|
Here you will find answers to the following questions:
|
Construction elements are selected with the following in mind: manufacturing procedure, products to be processed, room classes and economic efficiency.
3.E.1 Walls
It is possible to differentiate between two wall types: solid walls and stud construction walls.
Solid walls are usually external walls or structural partition walls. Solid walls are made of concrete or masonry and are characterised by high mechanical and thermal stability. They are therefore particularly suitable for rooms where operations involving heavy loads (stacker) or potentially-explosive substances (solvents) are carried out. A disadvantage of solid walls is that they offer a low degree of flexibility due to the high costs and mess involved during demolition/reconstruction.
Concrete or masonry walls may be untreated, plastered, painted, plastered and painted, papered, papered and painted or tiled depending on the requirements. Paints used in production areas and laboratories must be easy to clean, and must not release solvents or softening agents under working conditions. Latex and epoxy paints are most commonly used.
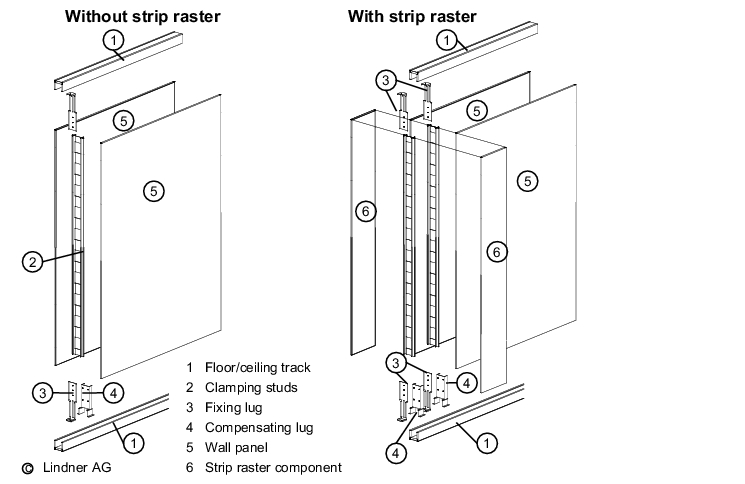
Stud constructions (see figure 3.E-1) may be used for partition walls, as well as facing for solid walls. Stud walls consist of a substructure (known as studs) and sandwich elements/panels. The materials most commonly used for the substructure are wood, aluminium, galvanised steel and stainless steel. Timber substructures should no longer be used in production areas due to the need for regular cleaning in these areas.
Sheeting materials used to cover the studs include plasterboard (painted, papered, tiled), laminated timber, powder-coated sheet steel, stainless steel, glass and plastics.
|
|
In order to accommodate GMP requirements regarding cleanability and prevention of contamination, the details for the various locations described below are always important, irrespective of the wall type used. To reduce the investment costs, it must be ensured that finishes required in sterile areas are not transferred to areas where requirements are less stringent. It is always worth investigating the options for simple cost-effective details with all parties involved (pharmacist, engineer, microbiologist, quality assurance).
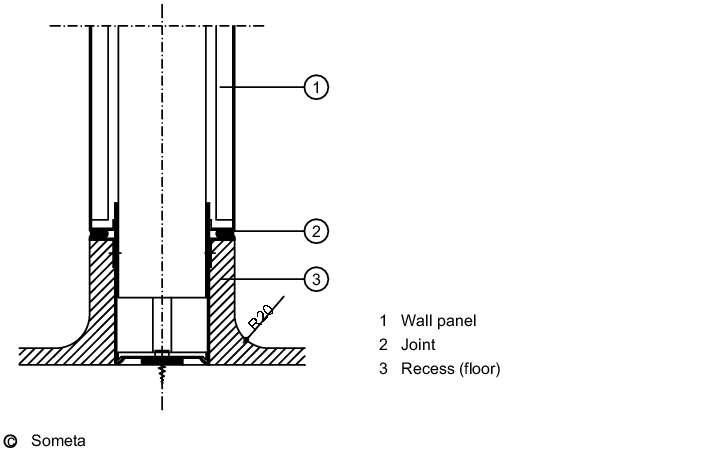
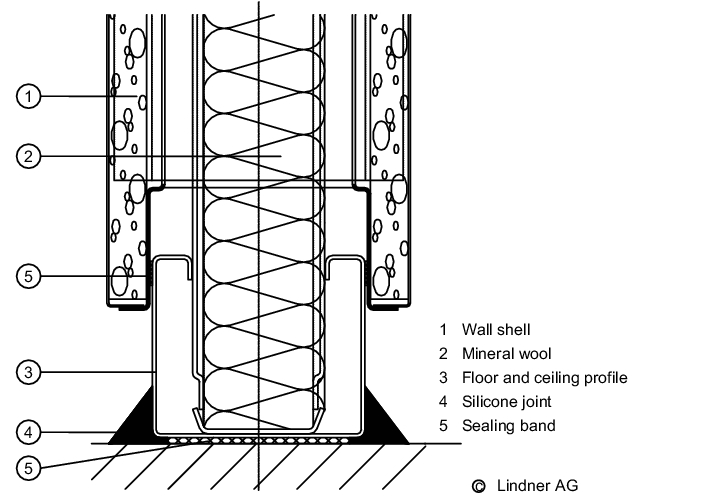
The junction between the wall and the floor (see figure 3.E-2) must be considered very carefully - particularly in sterile areas and in rooms where wet-cleaning is frequently carried out. The wall/floor detail must not incorporate a joint where dirt and micro-organisms could collect.
|
|
It must be leak-tight to prevent water penetrating the substructure or neighbouring rooms. A wall system must be chosen appropriate to the flooring used, and the junction between the wall and the floor must be easy to clean. For sterile areas and rooms where wet cleaning is frequently carried out, recessed junctions are indispensable, but in rooms with less stringent requirements, e.g. packaging operations, simpler versions are permissible (see figure 3.E-3)
|
|
.
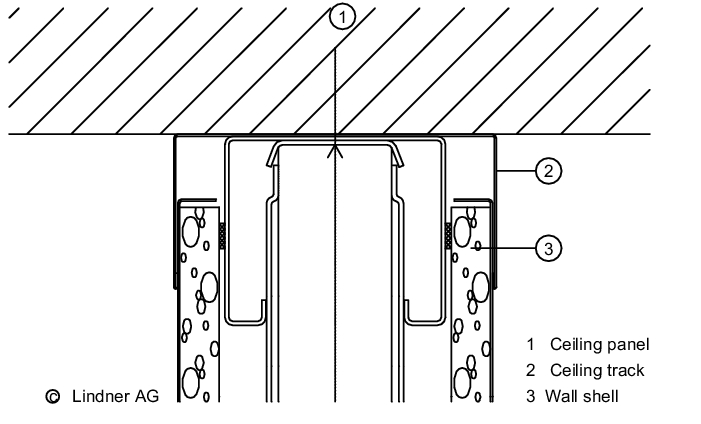
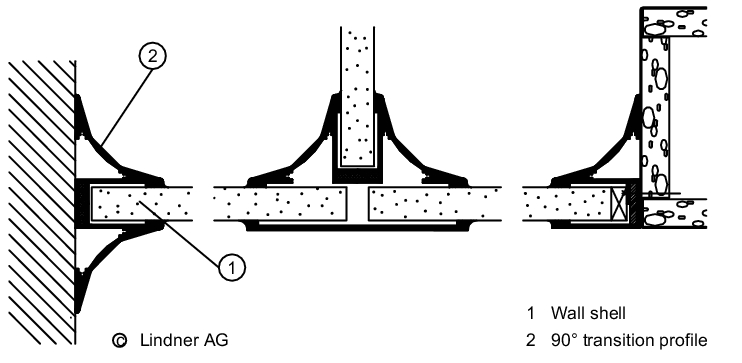
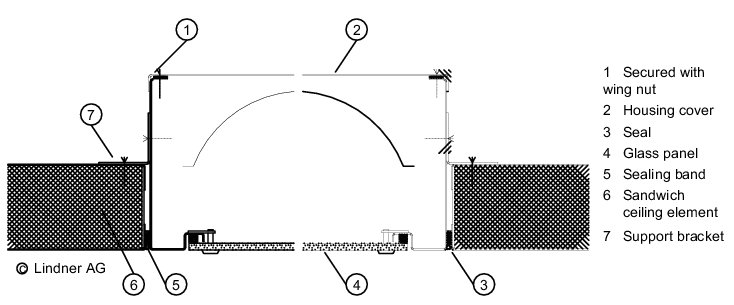
The wall/ceiling connection is important for preventing the entry of particles from adjacent areas (see figure 3.E-4).
|
|
The penetration of walls is necessary in all areas to connect utilities (pipes, electrical fixtures such as light switches and sockets, warning lamps, measuring instruments, etc.) from the technical areas to the production rooms. From the GMP standpoint, these points are particularly critical as they are a potential source of leaks, particle release and contamination - particularly if facings are also mounted. However, it is also important for flexibility to be able to make wall penetrations quickly, simply (without interfering with operations) and economically.
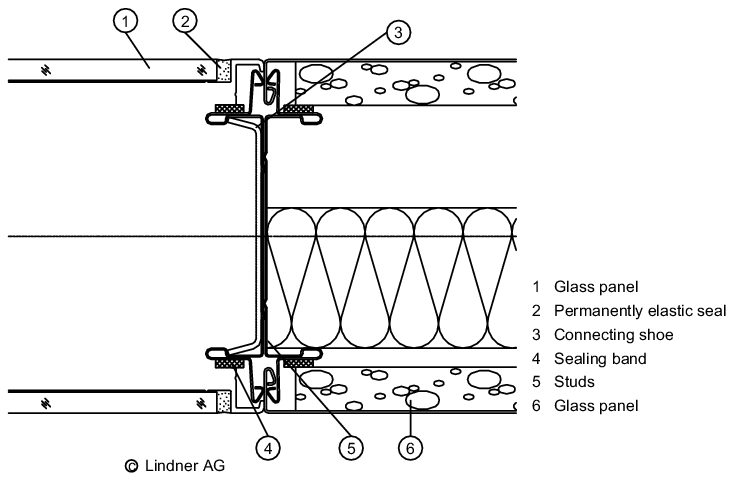
For constructional reasons, most stud walls have joints between the individual cladding elements. As a general rule, the fewer joints there are, the more complex and expensive the wall will be. To this end, a range of partition wall systems exist for sterile areas that incorporate fewer joints and allow windows and doors to be installed flush with the wall. An inexpensive alternative is plasterboard (requires no joints) with a suitable paint or coating.
However, this solution has limitations if it is necessary to apply high mechanical loads to the walls or if the rooms must be wet cleaned regularly. In sterile areas rounded corners are a further detail that can be used to make the cleaning of walls easier (see figure 3.E-5).
|
|
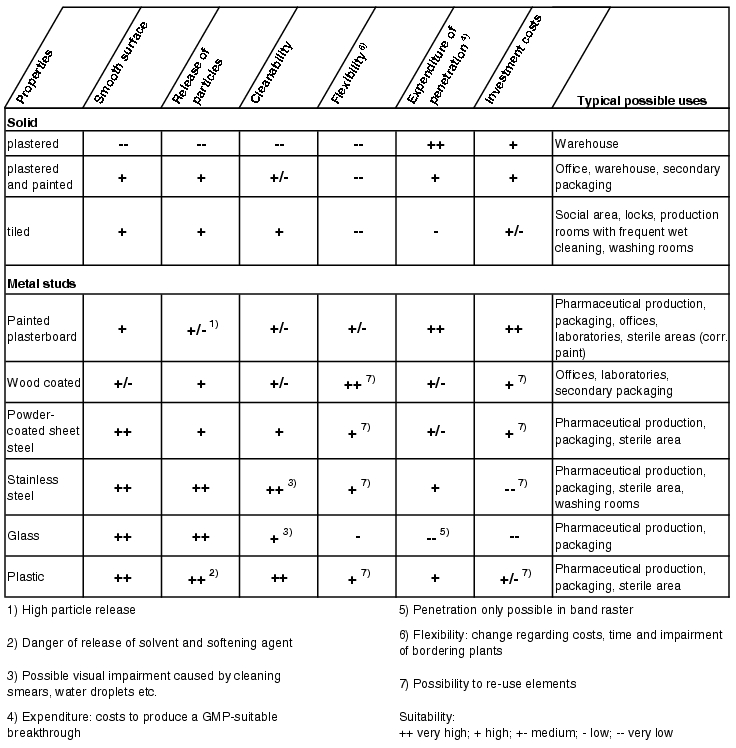
Figure 3.E-6 shows a selection of wall systems commonly used in pharmaceutical production with an assessment of their compatibility with GMP requirements.
|
|
3.E.2 Doors and windows
An important requirement for sterile areas is that it must be possible to install windows and doors flush with the walls without creating any surfaces or edges on which dust could gather.
|
|
Windows, while improving the visual appearance of a room, have the disadvantage that they must be cleaned more often than non-transparent elements. The flush installation of windows is a state-of-the-art approach employed in sterile areas. For the remaining pharmaceutical manufacturing areas this is also desirable but should not be adopted as the standard approach due to increased costs. Generally, windows to the outside must be kept sealed; emergency exit windows must have at least one seal; windows in external walls in sterile areas must be flush and fully integrated.
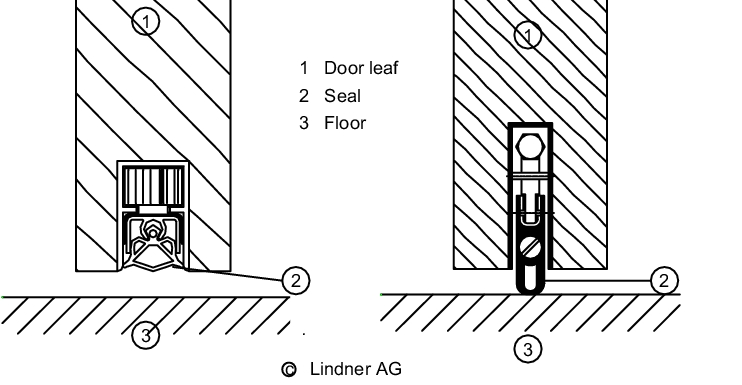
The above also applies for the flush installation of doors. In addition, care must be taken to ensure that windows, and hardware in the door leaves, are installed flush. Doors must generally be kept closed to maintain the pressure characteristics of the rooms and to avoid cross-contamination so it is recommended that doors are equipped with closers. Door thresholds must be avoided as they are difficult to clean. Interlocking doors must be used in locks. Doors may also be combined with access authorisation systems. For areas with special requirements, there are doors with inflatable gaskets or lip seals at the foot. Wooden doors should not be used in areas that have to be cleaned regularly. Powder-coated sheet steel, aluminium, stainless steel and plastic are all suitable materials.
|
|
A distinction is made between manually-operated and power-operated doors. Manually-operated doors are generally swing doors, also with two leaves (one active and one inactive), possibly equipped with a mechanical closer. These doors may easily be installed flush. Floor holes to accommodate locking bolts for the inactive door leaf are microbiologically critical.
Power-operated doors are advantageous in that they may be opened and closed automatically (via buttons or remote control) They are indispensable where automated transport equipment is used. The following types are commonly used:
- Sliding doors: Advantage: they save space. Disadvantages: the drive is difficult to clean and they are problematic where high positive pressure exists in the room. Sliding doors may only be used in production areas if the floor strip can be omitted. Areas of use: warehouse, pharmaceutical manufacturing, and packaging, but not in sterile areas.
- High-speed doors: (roll-up door or folding door) door made of plastic foil or lamellas. Advantages: space-saving, fast-opening. Disadvantages: plastic ages and is difficult to clean. Areas of use: Warehouse, pharmaceutical manufacturing and packaging, not in sterile areas.
- Swing doors: Advantage: they are easy to clean. Disadvantage: they take up space. Areas of use: pharmaceutical manufacturing including sterile areas.
3.E.3 Floors
Floors must meet many functional criteria: they must be easy to clean and conductive, mechanically, chemically and thermally resistant, smooth, non gas forming and also visually attractive, i.e. light in colour. Often nearly all of these criteria must be satisfied together. Flooring that meets all requirements does not exist, so the most suitable covering for the intended purpose must be selected. As an example, flooring that is subjected to a high degree of mechanical stress by the movement of stacking equipment, may be made of ceramic coverings with joints. In the sterile area, only seamless, smooth floors incorporating a recess can be considered.
Flooring consists of a substructure made of reinforced concrete, or precast concrete elements, and a covering. The latter may be made up of many individually-applied layers that ensure adhesion to the substructure, smooth out unevenness, bridge cracks, ensure conductivity and protect the floor against the penetration of dirt, water and micro-organisms.
Two types of floor finishes are used in pharmaceutical manufacturing and packaging: floors laid without seams and floors with seams. For seamless floors, a differentiation is made between floors with welded seams (e.g. PVC coatings) and coverings such as epoxy resin flooring or pharma terrazzo. In both cases, the standard of installation workmanship is decisive for the quality and durability of the floor.
An advantage of seamless floors is that they have a completely smooth surface, are easy to clean and are chemically-resistant. Disadvantages are their lack of both mechanical and thermal resistance. The surface must have sufficient surface rawness and must also be pore free. Places of use: sterile areas, standard production areas, PVC floors also in laboratory, social areas, and corridors used by personnel.
Where appropriately constructed, tiled floors or floors with ceramic coverings, have a high mechanical load capacity. Conductive versions of ceramic coverings are now available. The disadvantages of tiled floors are the number of joints and the difficulty of constructing openings for gullies or pipe bushings. It is now possible to face-grind tile coverings and then apply a sealing coat. Places of use: pharmaceutical production and packaging; wash rooms, social areas. In sterile areas, only to be used with a surface seal.
Figure 3.E-9 provides an overview of the advantages and disadvantages of various floor finishes.
|
Place of use |
Advantages |
Disadvantages |
|
|---|---|---|---|
|
Pharma terrazzo |
sterile areas, solids manufacturing, washing rooms |
Seamless, easy to clean, conductive, with recess, resistant to chemicals, easy to work with |
Mechanical and thermal resistance, costs, skid resistance |
|
Coating (epoxy/polyurethane) |
Packaging areas without stacker traffic, solids production, warehouse |
Seamless, easy to clean, conductive, cheaper than pharma terrazzo, resistant to chemicals, easy to work with |
Mechanical and thermal resistance, no recess, skid resistance |
|
Tiles, ceramic coverings |
Solids manufacturing, washing rooms, packaging areas with stacker traffic |
Chemical, thermal, mechanical resistance. Recess possible, conductive versions available |
Higher number of joints, complex installation |
|
Linoleum/PVC |
Laboratories, IPC, offices, corridors |
Easy to clean, recess, inexpensive |
Mechanical, chemical, thermal resistance |
|
Concrete, |
Warehouse, stacker driveways |
Mechanical resistance, inexpensive |
Difficult to clean |
The following details are especially critical for all floor finishes from the GMP standpoint:
- Building expansion joints, junctions between different floor finishes: although these construction features are generally unavoidable, they should be used as little as possible. If these elements have to be included, they must be detailed very carefully: slits, cracks, ruptures, inaccessible joints, sharp edges and corners must be avoided. Junctions must be levelled out evenly as otherwise the flooring may fail due to high wheel loads of stackers passing over uneven junctions. High-quality materials such as stainless steel strips and plastics with long-term durability (15 years) must be used.
- Gradients: The flooring must always be laid to falls in the direction of the floor inlet or centre of the room to allow cleaning waste water to drain off, and to ensure that it cannot gather under walls, in corners or under machines. In practice this apparently simple requirement may be more difficult to achieve, so particular care must be taken in laying the floor.
- Recesses: these are a suitable means of making cleaning easier and preventing the diffusion of water into walls and adjacent rooms. Recesses are wall skirtings, covered ideally with the same material as the floor and also seamlessly joined to it. They should have a radius of at least 2 cm. Recesses are also available for tiled floors.
3.E.4 Ceilings
The ceiling construction, in addition to sealing off a room from adjacent areas, has a significant role to play in ensuring a GMP-compliant supply of rooms. The ceiling accommodates lighting as well as supply and exhaust ventilation systems. In many cases, utilities are also supplied to rooms from above for cleaning and flexibility. Ceilings must satisfy the same requirements as walls: they must be smooth and easy to clean, and must not shed particles; they must also be capable of being sealed in sterile areas. [EU GMP Guideline, Appendix 1.24].
Depending on the building design, the ceiling may be suspended from the building structure or it may be solid (concrete). The building services (utilities, ventilation ducts and power cables, etc.) must always be outside the room in production and packaging areas, i.e. routed in a services zone above the ceiling. Services in laboratories and storage areas may also be exposed, providing that they are cleaned regularly. Unfinished ceilings, painted or plastered and painted, are also possible in pharmaceutical manufacturing and packaging areas, with the exception of laboratories, and excluding sterile production.
Suspended ceilings consist of a raster with screwed-on or inserted elements. Depending on the requirements, the raster is constructed from galvanised, powder-coated steel or stainless steel and sometimes wood in exceptional cases (office, warehouse). The ceiling elements consist of plasterboard, plastic, powder-coated metal, anodised or stainless steel. Perforated ceiling panels with insulating material on the upper face may be used for soundproofing in manufacturing and packaging operations. Care must be taken to ensure that the insulation is protected and cannot shed particles (e.g. shrink-wrapped in foil). Special sound-absorbing panels for sterile areas are available that can be mounted flush in the ceiling.
If services to the rooms are supplied from above, it is recommended that this service zone be large enough to be accessed from outside the production rooms. This is the only way to ensure that maintenance and repair may be carried out regularly without the production rooms being adversely affected. This requirement of the EU GMP Guideline [Appendix 1.33] should also be observed in non-sterile areas as this shortens cleaning times and downtimes. Particular attention must be paid to the air outlets and lighting. Other openings must be made as described in chapter 3.E.1 Walls).
|
|
|
Summary: Particular attention must be paid to the detailing of components when designing rooms. The room class must be considered when selecting materials and construction methods: the higher the room class, the more complex and expensive the construction will be. |