|
Here you will find answers to the following questions:
|
"Premises and manufacturing equipment shall be located, designed, constructed, adapted and maintained to suit the intended operations."[EC Directive 2003/94/EC, Article 8.1]. Equipment and rooms are the basic technical components of pharmaceutical production. From the GMP standpoint they have a great significance, being in contact with pharmaceutical products from the raw material stage through to the finished product. The design of rooms is crucial to the quality of the pharmaceutical products and also relates to the cost effectiveness of production - both directly (investment costs) and indirectly (e.g. flow of materials). Essentially, the rooms should be built of such high quality as necessary for the product quality and as simply and economically as possible.
A room inside a pharmaceutical production building performs the same kinds of tasks as a cell in an organism. Essentially, while performing the function of an envelope in which an individual processing step takes place - by isolating it from other processing steps that are running at the same time - it must also perform a range of other tasks: the supply of energy and utilities for the manufacturing process; the creation of suitable climatic conditions; and the supply and disposal of materials. The optimum location of the rooms and their arrangement in relation to one another, not only ensures the quality of production, but also greatly influences cost effectiveness.
3.B.1 Location, connection to other rooms
"Premises should be situated in an environment which [...] presents minimal risk of causing contamination of materials or products." [Chapter 3.1 EU GMP Guideline]. In addition to this requirement however, a suitable room location ensures an appropriate flow of materials [chapter 3.7, EU GMP Guideline] together with compatibility with other manufacturing procedures in other rooms. The arrangement of rooms in the building should facilitate a linear sequence of individual processing steps. This reduces the risk of confusion and the possibility that quality-determining manufacturing and control steps will be missed out. The arrangement of rooms in the sterile area plays an essential role in ensuring compliance with the various room classes (keyword: locks). Additionally, the choice of location is an equally decisive factor - not only in terms of size but also for supply to and disposal from the rooms.
|
Building features |
|
|---|---|
|
Building structure |
Construction: dimensions, materials, number and height of storeys, column grid, suspended ceilings, statics Building services: air-conditioning technology, utilities (compressed air, nitrogen, steam), range of water qualities |
|
Building use |
Effects of other production operations, e.g. via ventilation system (hormones, highly potent substances) Effects of persons: are special access arrangements necessary? |
When planning the arrangement of the rooms, in new buildings as well as conversions, the building features presented in figure 3.B-1 must also be considered in order to select suitable locations.
Two examples are described below for illustration:
- IPC laboratory: in a laboratory, visual controls must often be carried out and assessed (colour, friability, microbiological growth etc.). It is therefore recommended that rooms where these kinds of activities take place are located in areas where natural light is available.
- Granulation of highly potent substances: persons and other products are to be protected in this area. The building features must be considered when choosing the location: is it possible to prevent contamination using air-conditioning technology? Does the structure of the building make hermetic shielding of different areas from one another possible? Is it possible to include locks?
3.B.2 Size, area, height
The area and plan required for the overall production, control and storage of a product are the decisive factors rather than the actual size of the room itself. Only one batch of one product may be processed in a room at any time. (See chapter 11.J Prevention of cross-contamination.)
Oversized rooms are often partially used as storage areas for previous or subsequent orders. If rooms are too small, materials are often stored directly outside them, e.g. in the corridor. The risk of mix-up is high in both instances.
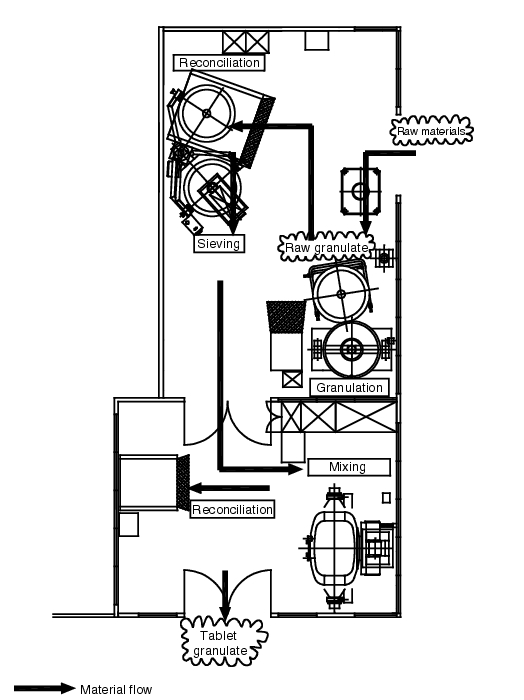
A suitable layout plan for rooms in which several production steps are processed in sequence is the prerequisite for preventing mix-ups through crossing-free logical structuring of operations [Chapter 3.8 EU GMP Guideline] (see figure 3.B-2).
|
|
The height of a room is largely determined by the building structure and the requirements of building legislation in relation to use. Room height must be considered in relation to factors such as the installation height of machines, the number and type of air vents as well as room classes.
3.B.3 Installation and supply of utilities
In addition to the type of installation that must facilitate easy cleaning [chapter 3.10, EU GMP Guideline], a process-oriented supply of utilities and energy must be aimed at, where the number and dimensioning of utilities must be harmonised with the process to achieve quality and economic efficiency.
Examples:
- Main ring (loop) for purified water: The dimensions of the pipes for purified water must be harmonised for all consumers in a loop, as the flow rate necessary to provide protection against microbial contamination may otherwise not be achieved, e.g. due to under-dimensioning.
- Power supply: the power supply may fluctuate within specific limits (400 V +/-20 V) or may fail completely. With sensitive measuring or control equipment, or analytical apparatus, these (permissible) tolerances may lead to inaccurate results: in such cases, appropriate technical measures must be taken (e.g. an emergency power supply).
3.B.4 Lighting, ventilation, air-conditioning
Air-conditioning provides for the supply of air to rooms and processes. As well as complying with the requirements of the guidelines for workplaces, the room air supply must be designed to meet the requirements of the products being manufactured. These range from variations in air humidity and air temperature to pressure differentials or flow directions between individual rooms. (See chapter 3.H.5 Design criteria for the ventilation of premises.)
The process air supply must satisfy only the requirements of the product or process as it is normally in direct contact with the products (e.g. fluid-bed drying). Depending on the requirements, it may be necessary for the conditioning of the air to be monitored using suitable recording systems.
The number and arrangement of lights must be determined in accordance with the guidelines for workplaces with respect to illumination levels. To facilitate easy cleaning and maintenance of light fittings, it is recommended, particularly in sterile areas, that these are accessible from the outside [chapter 3.10, EU GMP Guideline]. (See chapter 3.E.4 Ceilings.)
3.B.5 Hygienic construction
Production rooms must be designed in such a way that (uncontrolled) accumulation of dust and product particulate material cannot occur, or alternatively, that they should be easy to clean and disinfect (if necessary) [chapter 3.9, EU GMP Guideline]. This general requirement must be weighted according to the use of the room. The requirements are most stringent in the sterile area and are considerably lower in the packing area. Whatever the case, suitable protective measures must be taken to make sure that the ingress of animals and insects into the pharmaceutical production areas is prevented [chapter 3.4, EU GMP Guideline]. (See chapter 3.E Construction elements.)
3.B.6 Room book and layout
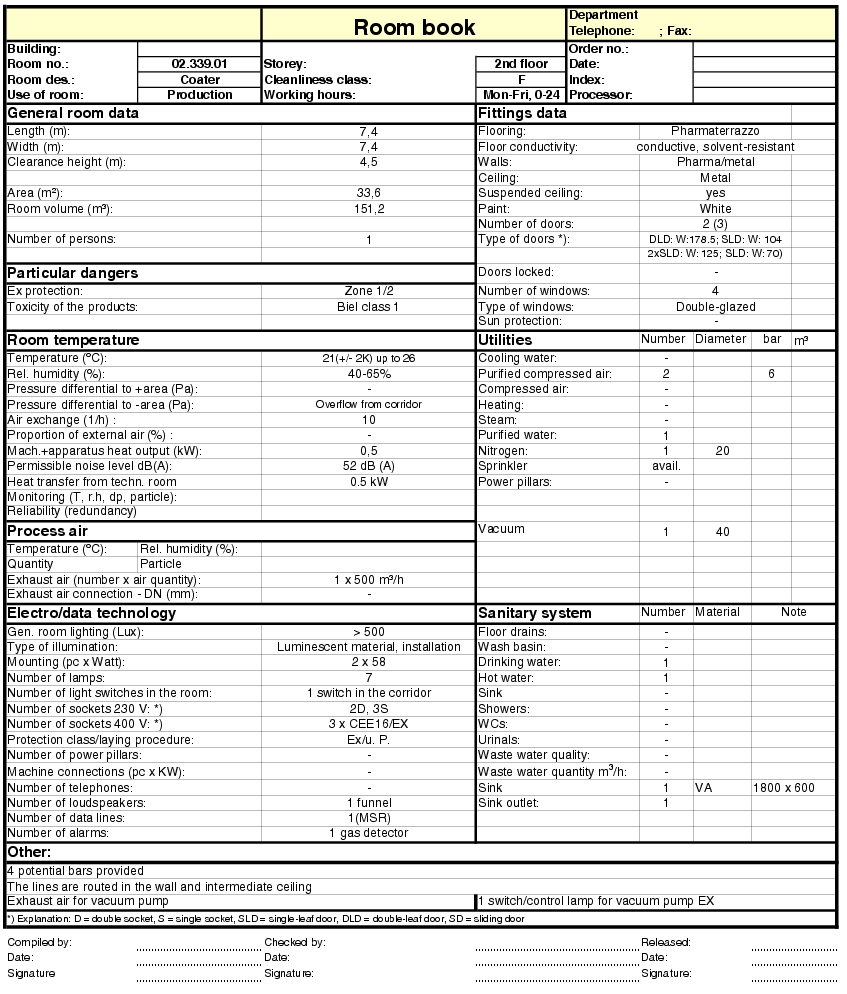
When designing rooms, in order to provide appropriate solutions in response to requirements, a range of data must be determined. The "room book", as it is referred to, is a useful document in which all data relevant to the room can be collected. Together with the layout, the room book presents the specification of a room. Both documents may also be used to illustrate company operations during inspections and also as the basis for the qualification of rooms.
The data for the room book (see figure 3.B-4) and layout design is determined from the requirements for use:
|
Requirements for use |
|---|
|
The following are derived from the product characteristics (temperature and sensitivity to moisture, warehouse stability, sensitivity to light, toxicity, etc.):
|
|
The following are derived from the manufacturing process (sterile, aseptic or non-sterile process, previous and subsequent processing steps and their connection, warehouse, packaging, control laboratory etc.):
|
|
Summary From the GMP standpoint, rooms have great significance as they are in contact with pharmaceutical products from the raw material to the finished product stage. The design of rooms affects the economic efficiency of production. The arrangement of rooms in the building should facilitate a linear sequence of individual processing steps. Production rooms must be designed in such a way that accumulation of dust and product particulate material cannot occur, or alternatively, so that they will be easy to clean and disinfect (if necessary). Rooms are specified by the room book and layout. |
|
|