Qualification of a packaging line
Just like all production equipment, a packaging line must also be qualified. The general process for a qualification is described in chapter 6 Qualification. The following pages contain examples for establishing qualification plans for a blister packaging line and the possible test items. These are only a sample and do not claim to be complete; the content of the qualification plans in particular must be adapted to the individual facility.
"Master qualification plan"
"Design qualification protocol"
"Design qualification report"
"Installation qualification protocol"
"Installation qualification report"
"Operational qualification protocol"
"Operational qualification report"
"Performance qualification protocol"
"Performance qualification report"
13.C.1 Master qualification plan
Company name |
Logo |
Master qualification plan |
Doc.no. |
Blister pack line |
Page x of y |
valid from: |
compiled
Name/function |
Date |
Signature |
Head of qualification |
|
|
checked
Head of project |
|
|
Head of process technology |
|
|
approved
Head of packaging company |
|
|
Head of quality assurance |
|
|
Archiving
Quality assurance (original) |
Packaging unit (copy) |
||
Document version index
Version |
Changes |
Date |
|
1 |
New facility |
see signature |
|
Contents
"Objectives"
"Responsibilities"
"Archiving"
"Short project description"
"Documents"
"Carrying out the qualification"
"Qualification phases"
"Test plans"
"Documentation of deficiencies"
"Result of the qualification levels"
Objectives
Only machines and facilities that are suitable for the specific purpose and for which this suitability can be demonstrated may be used to manufacture pharmaceutical products. The qualification of the machines and facilities is an adequate means of providing this proof. The various requirements and areas are described below with reference to the general master qualification plan (MQP) (SOP A1000-1). The objective of the qualification plan is to provide a summary of the qualification project.
Responsibilities
The allocated responsibilities for the various activities within the qualification project are listed below.
The personnel in the qualification team may be increased or modified, if necessary, within the qualification phase. If this is the case, the basic roles must still be guaranteed.
| Role |
Description of tasks |
Name |
Company |
|---|---|---|---|
Project manager |
|
Peter Luchs |
GMP-V |
Head of qualification |
|
Ludwig |
GMP-V |
Documentation representative |
|
Dieter Bär |
GMP-V |
Electrical engineering technician |
|
Edgar |
Electrotest |
Data |
|
Detlef Trudel |
Data-test |
Machine technician |
|
Martin Tappsig |
GMP-V |
Safety |
|
Siegfried Fuchs |
GMP-V |
Archiving
Qualification documents are archived in their original form in the packaging area (Q-Dok file) once they have received final authorisation from quality assurance. Copies of the qualification results are archived in the quality assurance, production management and packaging management departments. Quality assurance distributes the documents.
The documents are electronically stored under Q:\2003-01-VP\10003\ via the document management system.
Project planning
The objective is to start up the facility in October 2004. A rough outline is provided for information purposes.
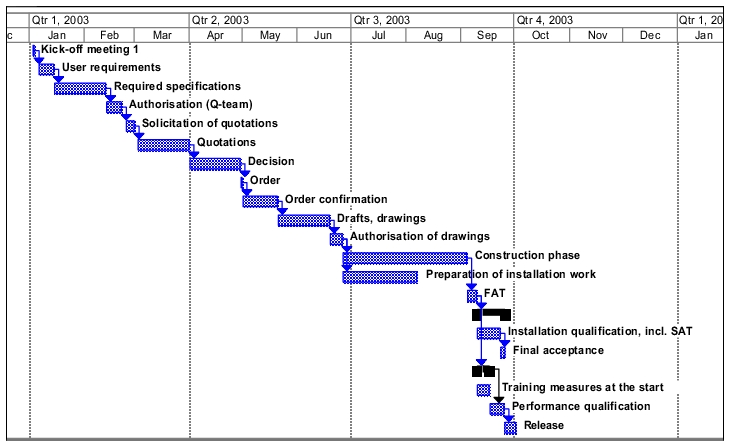
Short project description
There follows a short description of the project.
The detailed description of the requirements can be found in the corresponding documents. An additional packaging line must be installed to increase the capacity of solid, oral dosage forms. The entire modular facility consists of a thermoforming line, cartoning machine and bundling machine.
The machine is primarily for the processing of aluminium cover foil and aluminium thermoforming foil. It must be possible to process other standard foils, e.g. PVC foils. All the processing steps and process data must be able to be visualised during production. Data and fault messages must be documented.
The design must allow separation of the GMP and engineering areas.
It must be possible to observe the production process from outside.
The product feed must be realised in a simple way which protects the product and which is part of the facility equipment.
All surfaces that come into contact with the product must be of adequate quality, e.g. electro-polished stainless steel.
A more precise definition of the requirements is presented in the user requirements and/or required specifications.
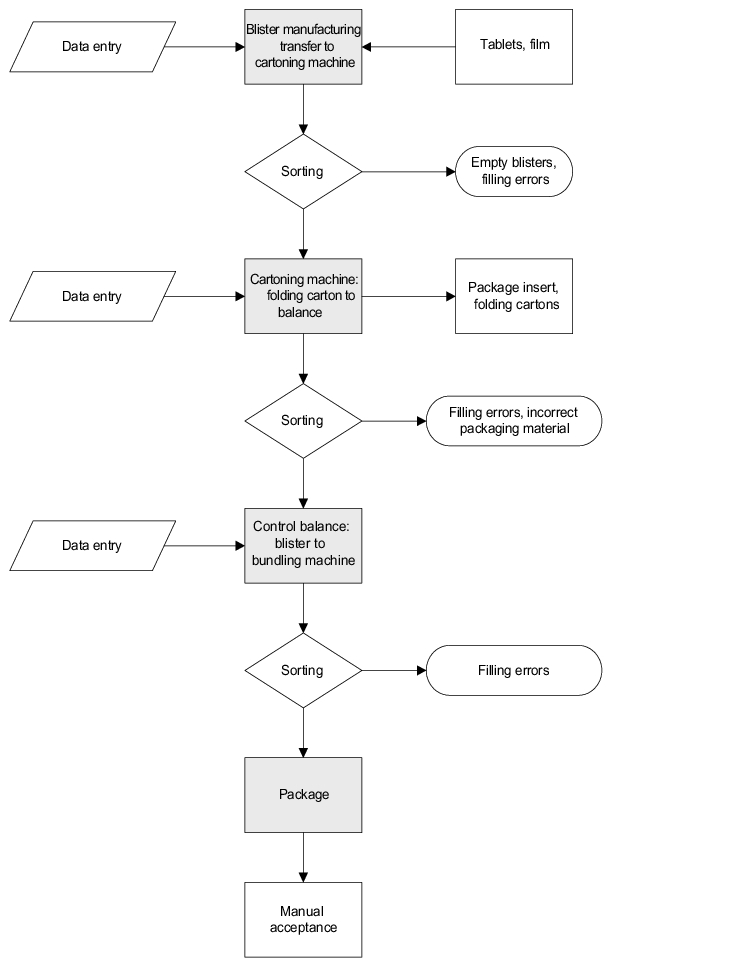
Documents
The following documents have already been compiled during the initial stages of the project or are available as a draft version.
| Document |
File path |
|---|---|
Qualification plan |
Q:\2003-01-VP\10003\MQP-01A01.pdf |
User requirements |
Q:\2003-01-VP\10003\DQP-LHA03.pdf |
Technical specifications |
Q:\2003-01-VP\10003\DQP-PH01A02.pdf |
DQ protocol |
Q:\2003-01-VP\10003\DQP-DQP01A02.pdf |
Carrying out the qualification
Qualification phases
The following phases will run throughout the entire qualification process:
- Design qualification
- Installation qualification
- Function qualification
- Performance qualification
Individual plans are compiled for these phases. The subsequent qualification phases cannot be commenced until the previous phase is complete. Completion is to be formally recognised by the authorisation and release of the report that is to be compiled. Deficiencies are permitted, insofar as they are not relevant to quality.
Plans and reports are compiled in the established format (SOP A1000-1).
Test plans
The test plans relating to the individual qualification levels are compiled and authorised accordingly by the persons responsible. The plans of the qualification levels contain references to the relevant authorised test plans.
Documentation of deficiencies
Deficiencies lists are to be drawn up to document deficiencies in a given qualification phase. The measures for remedying the deficiencies are also established in these lists. The deficiencies are evaluated and the measures are established by the qualification team. If the documented deficiencies are not relevant to quality, they may be passed on to the subsequent qualification phase.
The format for deficiencies lists is described below.
| Aspect |
Description |
Measure |
|---|---|---|
xxx |
xxx |
xxx |
Result of the qualification levels
A result is generated for the qualification activities based on the associated plan for the qualification level.
The level for the relevant qualification is: x complete. A deficiencies list is an integral part of the document and is included as part of the plan for the subsequent qualification level. |
The overall result of the qualification includes an evaluation of the performance qualification.
The level of operational qualification is x complete. A list of deficiencies has been finalised. The facility is qualified and released for production. All changes to the facility are subject to the change control procedure (SOP A1000CC05). |
13.C.2 Design qualification (DQ)
13.C.2.1 Design qualification protocol
Company name |
Logo |
Design qualification protocol |
Doc.no. |
Blister pack line |
Page x of y |
valid from: |
compiled
Name/function |
Date |
Signature |
Head of qualification |
|
|
checked
Head of project |
|
|
Head of process technology |
|
|
approved
Head of packaging unit |
|
|
Head of quality assurance |
|
|
Archiving
Quality assurance (original) |
Packaging unit(copy) |
||
Document modification index
Version |
Changes |
Date |
|
1 |
New facility |
see signature |
|
Contents
"Objectives"
"Responsibilities"
"Checks"
"Documents"
Objectives
The objective of the design qualification is to define the requirements on the facility to ensure that the facility is suitable for the intended production stage.. The associated specifications must be adapted to the precise facility in each individual case.
The requirements are documented in the user requirements and/or the technical specifications, which are used to draw up a quote.
Responsibilities
The responsibilities for this project are established in MQP.
Checks
Checks and activities are carried out as part of the design qualification:
Document check regarding presence, completeness and approval:
- User requirements
- Technical specifications
- Order, order confirmation
- Status of the supplier qualification
- Component list
- Installation plan
- Electro plans, pneumatics plans
- Project plan
Comparison of:
- Technical specification/order confirmation
Compilation of a:
- Risk analysis
- Deficiencies list (DQ)
Documents
The following documents have already been compiled during the initial stages of the project or are available as a draft version.
| Document |
File path |
|---|---|
Qualification plan |
Q:\2003-01-VP\10003\MQP-01A01.pdf |
User requirements |
Q:\2003-01-VP\10003\DQP-LHA03.pdf |
Technical specifications |
Q:\2003-01-VP\10003\DQP-PH01A02.pdf |
DQ protocol |
Q:\2003-01-VP\10003\DQP-DQP01A02.pdf |
13.C.2.2 Design qualification report
Company name |
Logo |
Design qualification report |
Doc.no. |
Blister pack line |
Page x of y |
valid from: |
compiled
Name/function |
Date |
Signature |
Head of qualification |
|
|
checked
Head of project |
|
|
Head of process technology |
|
|
approved
Head of packaging unit |
|
|
Head of quality assurance |
|
|
Archiving
Quality assurance (original) |
Packaging unit (copy) |
||
Document modification index
Version |
Changes |
Date |
|
1 |
New facility |
see signature |
|
Contents
"Documentation checking"
"Risk analysis"
"Framework documents"
"Deficiencies list"
"Result of the design qualification"
Documentation checking
The presence, completeness and approval have been checked in accordance with the qualification plan.
| Document |
Testing |
File path |
|---|---|---|
User requirements |
complies |
Q:\2003-01-VP\10003\DQP-LHA03.pdf |
Technical specifications |
complies |
Q:\2003-01-VP\10003\DQP-PH01A02.pdf |
Order, order confirmation |
complies |
Q:\2003-01-VP\10003\DQB-AUF04A03.pdf Q:\2003-01-VP\10003\DQB-AB01A01.pdf |
Status of the supplier qualification |
complies |
Q:\SuppQual\VP05.pdf |
Component list |
complies |
Q:\2003-01-VP\10003\DQB-FL01A01.pdf |
Installation plan |
complies |
Q:\2003-01-VP\10003\DQB-AUFPL01A03.pdf |
Electro plans, pneumatics plans |
complies see list of |
Q:\2003-01-VP\10003\DQB-ELEK03B01.pdf Q:\2003-01-VP\10003\DQB-PNEU02B04.pdf |
Project plan |
complies |
Q:\2003-01-VP\10003\DQP-PP02C02.pdf |
The technical specification and order confirmation were compared with the following result:
| Deviation |
Evaluation |
Measures |
|---|---|---|
Compressed air requirement is higher than defined |
Non-critical |
Available pressure is sufficient. Exceeded by 12 %. Overall capacity is not jeopardised. |
Control balance: change of type (successor) |
Non-critical |
Check as part of OQ |
Risk analysis
The following risk analysis was carried out and documented by the project team based on the available data and experiences.
| Company name |
Logo |
||||
|---|---|---|---|---|---|
| Design qualification report |
Doc.no. |
||||
| Blister pack line |
Page x of y |
||||
| No. |
Aspect/action |
Effect |
Solution |
Critical |
Measures |
Moulded foils |
|||||
1. |
End of foil |
Machine continues to run, products not packaged. |
Fault detected by sensor: Machine stop or prevention of start; Failure message |
yes |
OQ-check |
2. |
Adhesive parts |
Moulded foil adheres to packaging |
Sensor: no product supply, ejection of packages |
yes |
OQ-check |
3. |
Tear in aluminium moulded foil |
Machine continues operating |
Tear check: sensor to detect faults,interruption in product feed |
yes |
OQ-check |
Cover foils |
|||||
4. |
End of foil |
Machine continues to run, products not packaged. |
Fault detected by sensor: machine stop or prevention of start; failure message |
yes |
OQ-check |
5. |
Adhesive parts |
Cover foil adheres to packaging |
Sensor: no product supply, ejection of packages |
yes |
OQ-check |
6. |
Incorrect positioning of printed cover foil |
Package has incorrect printing |
Control of printed marks |
yes |
OQ-check |
Foil feed |
|||||
7. |
Foil inserted incorrectly |
unsuitable parameters, incorrect packages are rejected |
Monitoring of rollers |
yes |
OQ-check |
Heating station |
|||||
8. |
Temperature deviation |
Insufficient heating or damage to the moulded foil |
Sensors for automatic control of the actual temperatures (calibrated measuring system). Machine stop or prevention of start |
yes |
OQ-check, |
Moulding station |
|||||
9. |
Low compressed air level |
Incorrect shape of wells |
Monitoring of the compressed air, machine stops or start-up is prevented if there is negative pressure |
yes |
OQ-check, |
10. |
Insufficient cooling |
Moulded foil damaged |
Sensors for automatic control of the actual temperatures (calibrated measuring system). Machine stop or prevention of start |
yes |
OQ-check, |
11. |
Clamping force insufficient |
Incorrect shape of wells |
Readjustment of the clamping force |
yes |
OQ-check, |
Product supply |
|||||
12. |
Product feed not activated or in incorrect position |
Empty packages |
Linking of product feed (taking the pre-dosing option into consideration) and machine start-up in automatic mode |
yes |
OQ-check |
13. |
Holding tank empty |
Empty packages |
Content control for detection. Ejection of detected packages; Machine stop after defined number for production error |
yes |
OQ-check |
14. |
Imprecise positioning of the product in the wells |
Product is protruding |
Sensor: ejection of detected packages; Machine stop after defined number for production error |
yes |
OQ-check |
15. |
Incorrect, faulty products |
Size of product and packaging material are incompatible |
Content control, sensors |
yes |
OQ-check |
Content control |
|||||
16. |
Incorrect camera position |
Inadequate fault detection |
Permanent fault detection, .machine stop after defined number for production error |
yes |
OQ-check |
17. |
Incorrect alignment of the lens |
Inadequate fault detection |
Permanent fault detection, .machine stop after defined number for production error |
yes |
OQ-check |
18. |
Content control not activated |
No fault detection |
Failure message |
yes |
OQ-check |
19. |
Content control defective |
Inadequate fault detection |
Machine stop or prevention of start |
yes |
OQ-check |
Sealing station |
|||||
20. |
Anomalous sealing temperature display |
Inadequate seal |
Calibration of measuring system, test for tightness |
yes |
OQ-check, |
21. |
Anomalous sealing pressure display |
Inadequate seal |
Calibration of measuring system, test for tightness |
yes |
OQ-check, |
22. |
Imprecise positioning of the moulded and cover foil |
inadequate seal |
Alignment, tightness testing |
yes |
OQ-check |
Coding |
|||||
23. |
Imprecise positioning |
Coding in the wrong place or coding incomplete |
Alignment, sample test |
yes |
OQ-check |
24. |
Deviating temperature |
Inadequate coding or blister damage |
Sensors for automatic control of the actual temperatures (calibrated measuring system). Machine stop or prevention of start |
yes |
OQ-check Measuring point calibration |
25. |
Incorrect types |
Incorrect variable data |
Sample test |
yes |
not applicable |
Perforation station |
|||||
26. |
Blunt knife |
Inadequate perforation |
Sample test |
yes |
not applicable |
27. |
Incorrect tool |
Incorrect perforation |
Correction, sample test |
yes |
not applicable |
Die-cutting station |
|||||
28. |
Blunt knife |
Inadequate die cutting |
Sample test |
yes |
not applicable |
29. |
Incorrect tool |
Incorrect die cutting |
Correction, sample test |
yes |
not applicable |
Discharge |
|||||
30. |
Sucker damaged |
Undamaged product falls into collecting tray for damaged product |
Ejection controls |
yes |
OQ-check |
31. |
Ejection control fault |
No detection of damaged product, which is not ejected |
Failure message: machine stop or prevention of start |
yes |
OQ-check |
Sorting device |
|||||
32. |
Incorrect alignment |
Imprecise transfer to the subsequent process |
Sensor monitoring |
yes |
OQ-check |
33. |
Sensor fault |
Multiple transfer problems |
Failure message to control |
yes |
OQ-check |
Folding cartons |
|||||
34. |
Incorrect folding carton |
Incorrect folding carton |
Code reader |
yes |
OQ-check |
35. |
Low compressed air level |
Incorrect sorting, stacking, transfer |
Monitoring of the compressed air and vacuum control, machine stops or start-up is prevented |
yes |
OQ-check |
36. |
Incorrect number of blisters |
Under or overfilling of folding cartons |
Control balance, sensors |
yes |
OQ-check Balance calibration |
Folding carton coding |
|||||
37. |
Imprecise positioning |
Coding in the wrong place or coding incomplete |
Alignment, sample test |
yes |
not applicable |
38. |
Deviating temperature |
Inadequate coding or blister damage |
Sensors for automatic control of the actual temperatures (calibrated measuring system). Machine stop or prevention of start |
yes |
OQ-check Measuring point calibration |
39. |
Incorrect types |
Incorrect variable data |
Sample test |
yes |
not applicable |
Package insert |
|||||
40. |
Incorrect package leaflet |
Incorrect package insert |
Code reader |
yes |
OQ-check |
41. |
Package insert missing |
No package insert |
Code reader, lumat controls, ejection control, control balance |
yes |
OQ-check |
42. |
Packages closed incorrectly |
Packages open and damaged |
Sample control |
yes |
not applicable |
Code reader |
|||||
43. |
Code reader not activated |
No fault detection |
Failure message |
yes |
OQ-check |
44. |
Code reader faulty |
Inadequate fault detection |
Machine stop or prevention of start |
yes |
OQ-check |
45. |
Reject control, ejection control insufficient |
Incorrect packages |
Failure message, machine stops |
yes |
OQ-check |
Lumat reader |
|||||
46. |
Lumat reader not activated |
No fault detection |
Failure message |
yes |
OQ-check |
47. |
Lumat reader defective |
Inadequate fault detection |
Machine stop or prevention of start |
yes |
OQ-check |
Transfer, control balance |
|||||
48. |
Control balance not activated |
No fault detection |
Failure message |
yes |
OQ-check |
49. |
Control balance defective |
Inadequate fault detection |
In-process check |
yes |
not applicable |
50. |
Reject control, ejection control insufficient |
Incorrect packages |
In-process check |
yes |
not applicable |
Bundling machine |
|||||
51. |
Congestion transferring to bundling machine |
Congestion in the shrinking tunnel: package bundles are heated before the outlet |
Time recording and alarm if time is exceeded |
yes |
OQ-check |
52. |
Incorrect temperature |
Insufficient shrinking or thermal load |
Temperature monitoring |
yes |
OQ-check; |
53. |
Incorrect counting |
Incorrect number of packages in the bundle |
Sample test |
yes |
not applicable |
Control |
|||||
54. |
Incorrect function process and displays |
Machine functions faulty |
Testing |
yes |
OQ-check |
55. |
Incorrect parameters |
Machine functions faulty |
Testing |
yes |
not applicable |
56. |
Critical functions or parameters are not adequately protected (password) |
Settings made and modified by unauthorised (unqualified) personnel. |
Hierarchical password system |
yes |
OQ-check |
57. |
System validity lacking |
Reproducibility and product safety not guaranteed |
Testing |
yes |
IQ, OQ-check |
Energy supply |
|||||
58. |
Interruption in supply voltage |
Loss of data. Restarting results in incorrect packages |
Memory protected against zero voltage; noting and detecting positions and ejection volumes |
yes |
OQ-check |
Generalities |
|||||
59. |
Operator error |
Incorrect packages |
Training, operating procedures, displays, messages, documentation procedures |
yes |
not applicable |
Framework documents
| Document |
File path |
|---|---|
Qualification plan |
Q:\2003-01-VP\10003\MQP-01A01.pdf |
DQ protocol |
Q:\2003-01-VP\10003\DQP-DQP01A02.pdf |
DQ report |
Q:\2003-01-VP\10003\DQB-DQB01A02.pdf |
Deficiencies list
| Aspect |
Description |
Measure |
|---|---|---|
Component list |
Update after change is still outstanding. No effect on GMP aspects, therefore non-critical |
Update |
Pneumatics plan |
Agreed change still to be updated in drawing. No effect on GMP aspects, therefore non-critical |
Update |
Result of the design qualification
The checks and activities have been carried out in accordance with the specifications of the qualification plan for the design qualification.
The level of design qualification is x complete. A deficiencies list is an integral part of the document and is included as part of the plan for the installation qualification. |
13.C.3 Installation qualification (IQ)
13.C.3.1 Installation qualification protocol
Company name |
Logo |
Installation qualification protocol |
Doc.no. |
Blister pack line |
Page x of y |
valid from: |
compiles
Name/function |
Date |
Signature |
Head of qualification |
|
|
checked
Head of project |
|
|
Head of process technology |
|
|
approved
Head of packaging unit |
|
|
Head of quality assurance |
|
|
Archiving
Quality assurance (original) |
Packaging unit (copy) |
||
Document modification index
Version |
Changes |
Date |
|
1 |
New facility |
see signature |
|
Contents
"Objectives"
"Responsibilities"
"Checks"
"Documentation checking"
"Component checking"
"Test plans"
"Documents"
Objectives
The objective of the installation qualification is to document the correct implementation of the previously defined requirements for the manufacturing and assembly operations of the facility. The documentation contains information about identification of the overall scope of delivery of the pharmaceutical process equipment and confirmation that the supplied components correspond to the specifications that were defined during the draft phase.
Responsibilities
The responsibilities for this project are established in MQP.
Checks
Checks and activities are carried out as part of the installation qualification:
Documentation checking
Document check regarding presence, completeness and approval:
- DQ report
- DQ deficiencies list
- Installation drawing
- Certificates (ISO, CE, free from asbestos)
- Material specification of parts coming into contact with the product
- Calibration list: List of measuring points, calibration procedures, suitability of measuring instruments
- Technical documentation (maintenance schedule, user instructions, list of lubricants, list of filters, MSR list, pneumatics plan, media supply, equipment list, spare parts list)
- QA machine acceptance by the suppliers
- FAT records
Component checking
Check for the correct installation of the defined components:
- Completeness and conformity with the plans
- Pressure test (moulding station)
- Test of the direction of rotation (motors)
- Calibration (GMP-relevant measured sections for temperatures and pressures, balances (dynamic, static), camera systems, code readers; each with min./max./target value)
Check of aspects subject to CSV:
- Software: version number
- Plan for input/output, electrical diagram
- Function plan with parameter list
- Explanation of symbols and variables
- Back-up file
Test plans
The relevant test plans including the acceptance criteria and specifications for execution are in the documents listed below. The defined checks are carried out as part of the FAT. The various individual test plans are referred to in the FAT plan document.
| FAT |
File path |
|---|---|
Specification for execution |
Q:\QZ\FAT03A01.pdf |
FAT protocol |
Q:\2003-01-VP\10003\IQP-FAT01A01.pdf |
| Calibration |
File path |
|---|---|
Specification for execution |
Q:\Kalib\TEMP04D01.pdf |
Foil heating, upper plate |
Q:\2003-01-VP\10003\IQP-KAL01A02.pdf |
Foil heating, lower plate |
Q:\2003-01-VP\10003\IQP-KAL02A02.pdf |
Moulding station cooling |
Q:\2003-01-VP\10003\IQP-KAL03A02.pdf |
Moulding station heating |
Q:\2003-01-VP\10003\IQP-KAL04A02.pdf |
Perforation heating |
Q:\2003-01-VP\10003\IQP-KAL05A02.pdf |
Coding heating |
Q:\2003-01-VP\10003\IQP-KAL06A01.pdf |
Sealing pressure 1 |
Q:\2003-01-VP\10003\IQP-KAL01B01.pdf |
Sealing pressure 2 |
Q:\2003-01-VP\10003\IQP-KAL02B01.pdf |
Manometer |
Q:\Kalib\PRESSURE02E01.pdf |
| Component check |
File path |
|---|---|
Specification for execution |
Q:\QZ\COMP03A02.pdf |
Utilities |
Q:\2003-01-VP\10003\IQP-COMP01A01.pdf |
Electrics |
Q:\2003-01-VP\10003\IQP-COMP02A02.pdf |
Pneumatics |
Q:\2003-01-VP\10003\IQP-COMP03A03.pdf |
Overall configuration |
Q:\2003-01-VP\10003\IQP-COMP04A02.pdf |
| CSV analysis |
File path |
|---|---|
Specification for execution |
Q:\QZ\CSV04A01.pdf |
Testing |
Q:\2003-01-VP\10003\IQP-CSV01A03.pdf |
Documents
The following documents have already been compiled during the initial stages of the project or are available as a draft version.
| Document |
File path |
|---|---|
Qualification plan |
Q:\2003-01-VP\10003\MQP-01A01.pdf |
DQ protocol |
Q:\2003-01-VP\10003\DQP-DQP01A02.pdf |
DQ report |
Q:\2003-01-VP\10003\DQB-DQB01A02.pdf |
IQ protocol |
Q:\2003-01-VP\10003\IQP-IQP03A01.pdf |
13.C.3.2 Installation qualification report
Company name |
Logo |
Installation qualification report |
Doc.no. |
Blister pack line |
Page x of y |
valid from: |
compiled
Name/function |
Date |
Signature |
Head of qualification |
|
|
checked
Head of project |
|
|
Head of process technology |
|
|
approved
Head of packaging unit |
|
|
Head of quality assurance |
|
|
Archiving
Quality assurance (original) |
Packaging unit (copy) |
||
Document modification index
Version |
Changes |
Date |
|
1 |
New facility |
see signature |
|
Contents
"DQ deficiencies list"
"Checks"
"Documentation checking"
"Calibration"
"Component checks"
"Framework documents"
"Result of the installation qualification"
DQ deficiencies list
The following deficiencies have been documented as part of the design qualification. The current status has been checked.
| Document |
DQ status |
IQ status |
|---|---|---|
Component list |
Update after change is still outstanding. No effect on GMP aspects, therefore non-critical |
complete |
Pneumatics plan |
Agreed change still to be updated in drawing. No effect on GMP aspects, therefore non-critical |
complete |
Checks
Checks and activities are carried out as part of the installation qualification:
Documentation checking
The presence, completeness and approval have been checked in accordance with the qualification plan.
| Document |
Testing |
File path |
|---|---|---|
DQ report |
complies |
Q:\2003-01-VP\10003\DQB-DQB01A02.pdf |
DQ deficiencies list |
complies |
Q:\2003-01-VP\10003\DQB-DQB01A02.pdf |
Installation drawing |
complies |
Q:\2003-01-VP\10003\IQB-INSTZ02A03.pdf |
Certificates (ISO, CE, free from asbestos) |
complies |
Q:\2003-01-VP\10003\IQB-CERT01A03.pdf |
Material specification of parts coming into contact with the product |
complies |
Q:\2003-01-VP\10003\IQB-CERT02A02.pdf |
Calibration list: List of measuring points, calibration procedures, suitability of measuring instruments |
complies |
Q:\2003-01-VP\10003\IQP-CERT01K02.pdf |
| Document |
Testing |
File path |
|---|---|---|
Technical documentation (maintenance schedule, user instructions, list of lubricants, list of filters, MSR list, pneumatics plan, media supply, equipment list, spare parts list) |
complies see list of deficiencies |
Q:\2003-01-VP\10003\IQB-DOC05T01.pdf Q:\2003-01-VP\10003\IQB-DOC07T01.pdf Q:\2003-01-VP\10003\IQB-DOC08T01.pdf Q:\2003-01-VP\10003\IQB-DOC10T01.pdf Q:\2003-01-VP\10003\IQB-DOC12T01.pdf Q:\2003-01-VP\10003\IQB-DOC13T01.pdf Q:\2003-01-VP\10003\IQB-INST01A01.pdf Q:\2003-01-VP\10003\IQB-INST02A02.pdf Q:\2003-01-VP\10003\IQB-INST03A03.pdf Q:\2003-01-VP\10003\IQB-DOC22T01.pdf |
QA machine acceptance by the suppliers |
complies |
Q:\2003-01-VP\10003\IQB-DOC01QA01.pdf |
FAT records |
complies |
Q:\2003-01-VP\10003\IQB-FAT01A01.pdf Q:\2003-01-VP\10003\IQB-FAT02A01.pdf Q:\2003-01-VP\10003\IQB-FAT03A01.pdf Q:\2003-01-VP\10003\IQB-FAT04A01.pdf Q:\2003-01-VP\10003\IQB-FAT05A01.pdf Q:\2003-01-VP\10003\IQB-FAT06A01.pdf Q:\2003-01-VP\10003\IQB-FAT07A01.pdf Q:\2003-01-VP\10003\IQB-FAT08A01.pdf Q:\2003-01-VP\10003\IQB-FAT09A01.pdf Q:\2003-01-VP\10003\IQB-FAT10A01.pdf |
Aspects subject to CSV |
complies |
Q:\2003-01-VP\10003\IQB-CSV01D01.pdf |
Calibration
| Document |
Testing |
File path |
|---|---|---|
Film heating, upper plate |
complies |
Q:\2003-01-VP\10003\IQP-CAL01A02.pdf |
Film heating, lower plate |
complies |
Q:\2003-01-VP\10003\IQB-CAL02A02.pdf |
Moulding station cooling |
complies |
Q:\2003-01-VP\10003\IQB-CAL03A02.pdf |
Moulding station heating |
complies |
Q:\2003-01-VP\10003\IQB-CAL04A02.pdf |
Perforation heating |
complies |
Q:\2003-01-VP\10003\IQB-CAL05A02.pdf |
Coding heating |
complies |
Q:\2003-01-VP\10003\IQB-CAL06A01.pdf |
Sealing pressure 1 |
complies |
Q:\2003-01-VP\10003\IQB-CAL01B01.pdf |
Sealing pressure 2 |
complies |
Q:\2003-01-VP\10003\IQB-CAL02B01.pdf |
Manometer |
complies |
Q:\2003-01-VP\10003\IQB-CAL0D01.pdf |
Balances (dynamic, static) |
complies |
Q:\2003-01-VP\10003\IQB-CAL01W01.pdf |
Component checks
| Document |
Testing |
File path |
|---|---|---|
Code reader (see FAT) |
complies |
Q:\2003-01-VP\10003\IQB-FAT01B01.pdf |
Pressure vessel (see FAT) |
complies |
Q:\2003-01-VP\10003\IQB-FAT02B1.pdf |
Motor test (see FAT) |
complies |
Q:\2003-01-VP\10003\IQB-FAT03B01.pdf |
Utilities (see FAT) |
complies |
Q:\2003-01-VP\10003\IQB-FAT04B01.pdf |
Electrics (see FAT) |
complies |
Q:\2003-01-VP\10003\IQB-FAT05B01.pdf |
Pneumatics (see FAT) |
complies |
Q:\2003-01-VP\10003\IQB-FAT06B01.pdf |
Total configuration |
complies |
Q:\2003-01-VP\10003\IQB-FAT07B01.pdf |
Framework documents
| Document |
Testing |
|---|---|
Qualification plan |
Q:\2003-01-VP\10003\MQP-01A01.pdf |
DQ plan |
Q:\2003-01-VP\10003\DQP-DQP01A02.pdf |
DQ report |
Q:\2003-01-VP\10003\DQB-DQB01A02.pdf |
IQ plan |
Q:\2003-01-VP\10003\ICP-IQP03A01.pdf |
IQ report |
Q:\2003-01-VP\10003\ICB-ICB02A02.pdf |
IQ deficiencies list
The following deficiencies have been determined based on the checks.
| Aspect |
Description |
Measure |
|---|---|---|
Certificates (free from asbestos) |
Certificate to prove that there is no asbestos is still outstanding. Clarification from the vendor from his supplier. |
Update |
Technical documentation (maintenance schedule, spare parts list) |
Maintenance schedule and spare parts list are not current |
Update |
Total configuration (see FAT) |
Configuration could only be checked on individual machines. Total configuration is checked as part of OQ. |
Check in OQ |
Result of the installation qualification
The checks and activities have been carried out in accordance with the specifications of the qualification plan for the installation qualification.
The level of installation qualification is x complete. o not complete A deficiencies list is an integral part of the document and is included as part of the plan for the operational qualification. |
13.C.4 Operational qualification (OQ)
13.C.4.1 Operational qualification protocol
Company name |
Logo |
Operational qualification protocol |
Doc.no. |
Blister pack line |
Page x of y |
valid from: |
compiled
Name/function |
Date |
Signature |
Head of qualification |
|
|
checked
Head of project |
|
|
Head of process technology |
|
|
approved
Head of packaging unit |
|
|
Head of quality assurance |
|
|
Archiving
Quality assurance (original) |
Packaging unit (copy) |
||
Document modification index
Version |
Changes |
Date |
|
1 |
New facility |
see signature |
|
Contents
"Objectives"
"Responsibilities"
"Checks"
"Documentation checking"
"Function check"
"Test plans"
"Documents"
Objectives
The objective of the operational qualification is to check the correct operation of components in accordance with the operational specification.
Responsibilities
The responsibilities for this project are established in MQP.
Checks
Checks and activities are carried out as part of the operational qualification:
Documentation checking
Document check regarding presence, completeness and approval:
- IQ report
- IQ deficiencies list
- SOPs (cleaning, calibration, maintenance) available in at least draft format
Function check
Test for correct operation:
- Safety devices (emergency off, etc.)
- Test under normal and maximum conditions
- Checks of the reaction to malfunctions
- Aspects subject to CSV
Test plans
The relevant test plans including the acceptance criteria and specifications for execution are in the documents listed below.
| Safety devices |
File path |
|---|---|
Specification for execution |
Q:\SFK\SFK05A04.pdf |
Checks |
Q:\2003-01-VP\10003\OQP-SFK01A01.pdf |
The defined checks are carried out as part of the SAT. The various individual test plans are referred to in the SAT protocol document.
| SAT |
File path |
|---|---|
Specification for execution |
Q:\QZ\SAT02A01.pdf |
SAT protocol |
Q:\2003-01-VP\10003\OQP-SAT01A01.pdf |
| Check of the reaction to malfunctions |
File path |
|---|---|
Specification for execution |
Q:\QZ\STOER03A02.pdf |
Check of splice point control (moulded foil) |
Q:\2003-01-VP\10003\OQP-STR01A01.pdf |
Check of splice point control (cover foil) |
Q:\2003-01-VP\10003\OQP-STR02A01.pdf |
Aluminium crack control check |
Q:\2003-01-VP\10003\OQP-STR03A01.pdf |
Check of end of foil control (moulded foil) |
Q:\2003-01-VP\10003\OQP-STR04A01.pdf |
Check of end of foil control (cover foil) |
Q:\2003-01-VP\10003\OQP-STR05A01.pdf |
Temperature control (lower heating plate) |
Q:\2003-01-VP\10003\OQP-STR06A01.pdf |
Temperature control (upper heating plate) |
Q:\2003-01-VP\10003\OQP-STR07A01.pdf |
Temperature control (sealing temperature) |
Q:\2003-01-VP\10003\OQP-STR08A01.pdf |
Temperature control (coding) |
Q:\2003-01-VP\10003\OQP-STR09A01.pdf |
Temperature control (perforation) |
Q:\2003-01-VP\10003\OQP-STR10A01.pdf |
Temperature control (moulding station cooling) |
Q:\2003-01-VP\10003\OQP-STR11A01.pdf |
Content control check |
Q:\2003-01-VP\10003\OQP-STR12A01.pdf |
Packaging rejection check (under sealer roller during machine stop) |
Q:\2003-01-VP\10003\OQP-STR13A01.pdf |
Machine stop (production error) |
Q:\2003-01-VP\10003\OQP-STR14A01.pdf |
Ejection control check |
Q:\2003-01-VP\10003\OQP-STR15A01.pdf |
Transfer control check |
Q:\2003-01-VP\10003\OQP-STR16A01.pdf |
Compressed air control check |
Q:\2003-01-VP\10003\OQP-STR17A01.pdf |
Vacuum control check |
Q:\2003-01-VP\10003\OQP-STR18A01.pdf |
Interruption of power supply check |
Q:\2003-01-VP\10003\OQP-STR19A01.pdf |
Triggering of motor protecting switch |
Q:\2003-01-VP\10003\OQP-STR20A01.pdf |
Print mark control outer web sensor check |
Q:\2003-01-VP\10003\OQP-STR21A01.pdf |
Leaflet feed check |
Q:\2003-01-VP\10003\OQP-STR22A01.pdf |
Folding carton feed check |
Q:\2003-01-VP\10003\OQP-STR23A01.pdf |
Incorrect package sorting check |
Q:\2003-01-VP\10003\OQP-STR24A01.pdf |
Blister stack check |
Q:\2003-01-VP\10003\OQP-STR25A01.pdf |
Overload check |
Q:\2003-01-VP\10003\OQP-STR26A01.pdf |
Code reader check (leaflet) |
Q:\2003-01-VP\10003\OQP-STR27A01.pdf |
Code reader check (folding carton) |
Q:\2003-01-VP\10003\OQP-STR28A01.pdf |
Lumat check |
Q:\2003-01-VP\10003\OQP-STR29A01.pdf |
Blister pack control |
Q:\2003-01-VP\10003\OQP-STR30A01.pdf |
Folding carton coding control |
Q:\2003-01-VP\10003\OQP-STR31A01.pdf |
Folding carton closure control |
Q:\2003-01-VP\10003\OQP-STR32A01.pdf |
Control (product in folding carton) |
Q:\2003-01-VP\10003\OQP-STR33A01.pdf |
| CSV analysis |
File path |
|---|---|
Specification for execution |
Q:\QZ\CSV04A01.pdf |
Testing |
Q:\2003-01-VP\10003\OQP-CSV02A02.pdf |
Documents
The following documents have already been compiled during the initial stages of the project or are available as a draft version.
| Document |
File path |
|---|---|
Qualification plan |
Q:\2003-01-VP\10003\MQP-01A01.pdf |
DQ protocol |
Q:\2003-01-VP\10003\DQP-DQP01A02.pdf |
DQ report |
Q:\2003-01-VP\10003\DQB-DQB01A02.pdf |
IQ protocol |
Q:\2003-01-VP\10003\IQP-IQP03A01.pdf |
IQ report |
Q:\2003-01-VP\10003\ICB-ICB02A02.pdf |
OQ protocol |
Q:\2003-01-VP\10003\OQP-OQP02A01.pdf |
13.C.4.2 Operational qualification report
Company name |
Logo |
Operational qualification report |
Doc.no. |
Blister pack line |
Page x of y |
valid from: |
compiled
Name/function |
Date |
Signature |
Head of qualification |
|
|
checked
Head of project |
|
|
Head of process technology |
|
|
approved
Head of packaging unit |
|
|
Head of quality assurance |
|
|
Archiving
Quality assurance (original) |
Packaging unit (copy) |
||
Document modification index
Version |
Changes |
Date |
|
1 |
New facility |
see signature |
|
Contents
"IQ deficiencies list"
"Checks"
"Documentation checking"
"Function checks"
"Framework document"
"OQ deficiencies list"
"Result of the operational qualification"
IQ deficiencies list
The following deficiencies have been documented as part of the installation qualification. The current status has been checked.
| Aspect |
IQ status |
OQ status |
|---|---|---|
Certificates |
Certificate to prove that there is no asbestos is still outstanding. Clarification from the vendor from his supplier. |
complete |
Technical documentation (maintenance schedule, spare parts list) |
Maintenance schedule and spare parts list are not current |
complete |
Total configuration (see FAT) |
Configuration could only be checked on individual machines. Total configuration is checked as part of OQ. |
complete |
Checks
Checks and activities are carried out as part of the operational qualification:
Documentation checking
The presence, completeness and approval have been checked in accordance with the qualification plan.
| Document |
Testing |
File path |
|---|---|---|
IQ report |
complies |
Q:\2003-01-VP\10003\ICB-ICB02A02.pdf |
IQ deficiencies list |
complies |
Q:\2003-01-VP\10003\ICB-ICB02A02.pdf |
SOP: |
complies |
|
Maintenance Calibration Cleaning |
SOP T1111-1draft SOP T1111-15draft SOP R1003-57draft |
Function checks
| Safety devices |
Testing |
File path |
|---|---|---|
Analyses |
complies |
Q:\2003-01-VP\10003\OQP-SFK01A01.pdf |
| SAT |
Testing |
File path |
|---|---|---|
SAT records |
complies |
Q:\2003-01-VP\10003\OQB-SAT01A02.pdf Q:\2003-01-VP\10003\OQB-SAT02A02.pdf Q:\2003-01-VP\10003\OQB-SAT03A03.pdf Q:\2003-01-VP\10003\OQB-SAT04A01.pdf Q:\2003-01-VP\10003\OQB-SAT05A02.pdf Q:\2003-01-VP\10003\OQB-SAT06A01.pdf Q:\2003-01-VP\10003\OQB-SAT07A01.pdf |
| Test under normal/maximum conditions |
Testing |
File path |
|---|---|---|
Specification for execution |
complies |
Q:\QZ\NORM04Q03.pdf |
Test of automatic operation (normal conditions) |
complies |
Q:\2003-01-VP\10003\OQB-NOR01A02.pdf |
Test of automatic operation (maximum conditions) |
complies |
Q:\2003-01-VP\10003\OQB-NOR02A02.pdf |
Test of inching operation (normal conditions) |
complies |
Q:\2003-01-VP\10003\OQB-NOR03A02.pdf |
Test of inching operation (maximum conditions) |
complies |
Q:\2003-01-VP\10003\OQB-NOR04A02.pdf |
Test of set-up operation |
complies |
Q:\2003-01-VP\10003\OQB-NOR05A02.pdf |
Test of manual operation |
complies |
Q:\2003-01-VP\10003\OQB-NOR06A02.pdf |
Timer test |
complies |
Q:\2003-01-VP\10003\OQB-NOR07A02.pdf |
Check of production data collection |
complies |
Q:\2003-01-VP\10003\OQB-NOR08A02.pdf |
Print mark control check |
complies |
Q:\2003-01-VP\10003\OQB-NOR09A02.pdf |
Compressed air filter check (moulding station) |
complies |
Q:\2003-01-VP\10003\OQB-NOR10A02.pdf |
Pre-dosage check |
complies |
Q:\2003-01-VP\10003\OQB-NOR11A02.pdf |
Leaflet feed check |
complies |
Q:\2003-01-VP\10003\OQB-NOR12A02.pdf |
Folding carton feed check |
complies |
Q:\2003-01-VP\10003\OQB-NOR13A02.pdf |
Boxing check |
complies |
Q:\2003-01-VP\10003\OQB-NOR14A02.pdf |
Balance check |
complies |
Q:\2003-01-VP\10003\OQB-NOR15A02.pdf |
Bundling machine check |
complies |
Q:\2003-01-VP\10003\OQB-NOR16A02.pdf |
| Check of the reaction to malfunctions |
Testing |
File path |
|---|---|---|
Specification for execution |
complies |
Q:\QZ\STOER03A02.pdf |
Check of splice point control (moulded foil) |
complies |
Q:\2003-01-VP\10003\OQB-STR01A01.pdf |
Check of splice point control (cover foil) |
complies |
Q:\2003-01-VP\10003\OQB-STR02A01.pdf |
Aluminium crack control check |
complies |
Q:\2003-01-VP\10003\OQB-STR03A01.pdf |
Check of end of foil control (moulded foil) |
complies |
Q:\2003-01-VP\10003\OQB-STR04A01.pdf |
Check of end of foil control (cover foil) |
complies |
Q:\2003-01-VP\10003\OQB-STR05A01.pdf |
Temperature control (lower heating plate) |
complies |
Q:\2003-01-VP\10003\OQB-STR06A01.pdf |
Temperature control (upper heating plate) |
complies |
Q:\2003-01-VP\10003\OQB-STR07A01.pdf |
Temperature control |
complies |
Q:\2003-01-VP\10003\OQB-STR08A01.pdf |
Temperature control (coding) |
complies |
Q:\2003-01-VP\10003\OQB-STR09A01.pdf |
Temperature control (perforation) |
complies |
Q:\2003-01-VP\10003\OQB-STR10A01.pdf |
Temperature control (moulding station cooling) |
complies |
Q:\2003-01-VP\10003\OQB-STR11A01.pdf |
Content control check |
complies |
Q:\2003-01-VP\10003\OQB-STR12A01.pdf |
Packaging rejection check (under sealer roller during machine stop) |
complies |
Q:\2003-01-VP\10003\OQB-STR13A01.pdf |
Machine stop (production error) |
complies |
Q:\2003-01-VP\10003\OQB-STR14A01.pdf |
Ejection control check |
complies |
Q:\2003-01-VP\10003\OQB-STR15A01.pdf |
Transfer control check |
complies |
Q:\2003-01-VP\10003\OQB-STR16A01.pdf |
Compressed air control check |
complies |
Q:\2003-01-VP\10003\OQB-STR17A01.pdf |
Vacuum control check |
complies |
Q:\2003-01-VP\10003\OQB-STR18A01.pdf |
Interruption of power supply check |
complies |
Q:\2003-01-VP\10003\OQB-STR19A01.pdf |
Triggering of motor protecting switch |
complies |
Q:\2003-01-VP\10003\OQB-STR20A01.pdf |
Print mark control outer web sensor check |
complies |
Q:\2003-01-VP\10003\OQB-STR21A01.pdf |
Leaflet feed check |
complies |
Q:\2003-01-VP\10003\OQB-STR22A01.pdf |
Folding carton feed check |
complies |
Q:\2003-01-VP\10003\OQB-STR23A01.pdf |
Incorrect package sorting check |
complies |
Q:\2003-01-VP\10003\OQB-STR24A01.pdf |
Blister stack check |
complies |
Q:\2003-01-VP\10003\OQB-STR25A01.pdf |
Overload check |
complies |
Q:\2003-01-VP\10003\OQB-STR26A01.pdf |
Code reader check (leaflet) |
complies |
Q:\2003-01-VP\10003\OQB-STR27A01.pdf |
Code reader check (folding carton) |
complies |
Q:\2003-01-VP\10003\OQB-STR28A01.pdf |
Lumat check |
complies |
Q:\2003-01-VP\10003\OQB-STR29A01.pdf |
Blister pack control |
complies |
Q:\2003-01-VP\10003\OQB-STR30A01.pdf |
Folding carton coding control |
complies |
Q:\2003-01-VP\10003\OQB-STR31A01.pdf |
Folding carton closure control |
complies |
Q:\2003-01-VP\10003\OQB-STR32A01.pdf |
Control (product in folding carton) |
complies |
Q:\2003-01-VP\10003\OQB-STR33A01.pdf |
| CSV test |
Testing |
File path |
|---|---|---|
Testing |
complies |
Q:\2003-01-VP\10003\OQB-CSV02A02.pdf |
Framework document
| Document |
File path |
|---|---|
Qualification plan |
Q:\2003-01-VP\10003\MQP-01A01.pdf |
DQ protocol |
Q:\2003-01-VP\10003\DQP-DQP01A02.pdf |
DQ report |
Q:\2003-01-VP\10003\DQB-DQB01A02.pdf |
IQ protocol |
Q:\2003-01-VP\10003\ICP-IQP03A01.pdf |
IQ report |
Q:\2003-01-VP\10003\ICB-ICB02A02.pdf |
OQ protocol |
Q:\2003-01-VP\10003\OQP-OQP02A01.pdf |
OQ deficiencies list
The following deficiencies have been determined based on the test/checks.
| Aspect |
Description |
Measure |
|---|---|---|
not applicable |
not applicable |
not applicable |
Result of the operational qualification
The checks and activities have been carried out in accordance with the specifications of the qualification plan for the operational qualification.
The level of operational qualification is x complete o not complete A deficiencies list is an integral part of the document and is included as part of the plan for the operational qualification. |
13.C.5 Performance qualification (PQ)
13.C.5.1 Performance qualification protocol
Company name |
Logo |
Performance qualification protocol |
Doc.no. |
Blister pack line |
Page x of y |
valid from: |
compiled
Name/function |
Date |
Signature |
Head of qualification |
|
|
checked
Head of project |
|
|
Head of process technology |
|
|
approved
Head of packaging unit |
|
|
Head of quality assurance |
|
|
Archiving
Quality assurance (original) |
Packaging unit (copy) |
||
Document modification index
Version |
Changes |
Date |
|
1 |
New facility |
see signature |
|
Contents
"Objectives"
"Responsibilities"
"Checks"
"Documentation checking"
"Function check"
"Test plans"
"Documents"
Objectives
The objective of the performance qualification is to check the correct operation of the facility in accordance with the technical specifications.
Responsibilities
The responsibilities for this project are established in MQP.
Checks
Checks and activities are carried out as part of the performance qualification:
Documentation checking
Document check regarding presence, completeness and approval:
- OQ report
- OQ deficiencies list
- SOPs (cleaning, calibration, maintenance)
- Personnel training
Function check
The performance tests are carried out essentially for blister thickness and running characteristics in the upper, middle and lower adjustment ranges of the facility (temperature, pressure, speed) which are defined in the technical specifications. The tests use the target formats for contents and packaging material (folding carton, leaflets) that are established in the technical specifications.
Test plans
The relevant test plans including the acceptance criteria and specifications for execution are in the documents listed below. The placebo batches used for the tests are documented in the records.
| Tests |
File path |
|---|---|
Specification for execution |
Q:\QZ\PQ02Q03.pdf |
Format 1 (min, max. average), packaging material format 1 |
Q:\2003-01-VP\10003\PQP-T01A01.pdf |
Format 2 (min, max. average), |
Q:\2003-01-VP\10003\PQP-T02A02.pdf |
Format 2 (min, max. average), |
Q:\2003-01-VP\10003\PQP-T03A01.pdf |
Format 1 (min, max. average), |
Q:\2003-01-VP\10003\PQP-T04A02.pdf |
Control systems check |
Q:\2003-01-VP\10003\PQP-T05A03.pdf |
Documents
The following documents have already been compiled during the initial stages of the project or are available as a draft version.
| Document |
File path |
|---|---|
Qualification plan |
Q:\2003-01-VP\10003\MQP-01A01.pdf |
DQ protocol |
Q:\2003-01-VP\10003\DQP-DQP01A02.pdf |
DQ report |
Q:\2003-01-VP\10003\DQB-DQB01A02.pdf |
IQ protocol |
Q:\2003-01-VP\10003\IQP-IQP03A01.pdf |
IQ report |
Q:\2003-01-VP\10003\ICB-ICB02A02.pdf |
OQ protocol |
Q:\2003-01-VP\10003\OQP-OQP02A01.pdf |
OQ report |
Q:\2003-01-VP\10003\OQB-OQB02A02.pdf |
13.C.5.2 Performance qualification report
Company name |
Logo |
Performance qualification report |
Doc.no. |
Blister pack line |
Page x of y |
valid from: |
Name/function |
Date |
Signature |
Head of qualification |
checked
Head of project |
|
|
Head of process technology |
|
|
approved
Head of packaging unit |
|
|
Head of quality assurance |
|
|
Archiving
Quality assurance (original) |
Packaging unit (copy) |
||
Document modification index
Version |
Changes |
Date |
|
1 |
New facility |
see signature |
|
Contents
"OQ deficiencies list"
"Tests"
"Documentation checking"
"Function checks"
"Framework document"
"Result of the operational qualification"
"PQ deficiencies list"
"Result of the performance qualification"
OQ deficiencies list
No deficiencies have been determined based on the tests.
| Document |
File path |
Document |
|---|---|---|
not applicable |
not applicable |
not applicable |
Tests
Tests and activities are carried out as part of the operational qualification:
Documentation checking
The presence, completeness and approval have been checked in accordance with the qualification plan.
| Document |
Testing |
File path |
|---|---|---|
OQ report |
complies |
Q:\2003-01-VP\10003\OQB-OQB02A02.pdf |
IQ deficiencies list |
complies |
Q:\2003-01-VP\10003\OQB-OQB02A02.pdf |
SOP: Maintenance Calibration Cleaning |
complies |
SOP T1111-1 SOP T1111-15 SOP R1003-57 |
Personnel training |
complies |
Q:\TRAIN\VP\1753A1.pdf |
Function checks
| Aspects |
Testing |
File path |
|---|---|---|
Format 1 (min, max. average), packaging material format 1 |
complies |
Q:\2003-01-VP\10003\PQB-T01A01.pdf |
Format 2 (min, max. average), packaging material format 1 |
complies |
Q:\2003-01-VP\10003\PQB-T02A02.pdf |
Format 2 (min, max. average), packaging material format 2 |
complies |
Q:\2003-01-VP\10003\PQB-T03A01.pdf |
Format 1 (min, max. average), packaging material format 2 |
complies |
Q:\2003-01-VP\10003\PQB-T04A02.pdf |
Control systems check |
complies |
Q:\2003-01-VP\10003\PQB-T05A03.pdf |
Framework document
| Document |
File path |
|---|---|
Qualification plan |
Q:\2003-01-VP\10003\MQP-01A01.pdf |
DQ protocol |
Q:\2003-01-VP\10003\DQP-DQP01A02.pdf |
DQ report |
Q:\2003-01-VP\10003\DQB-DQB01A02.pdf |
IQ protocol |
Q:\2003-01-VP\10003\ICP-IQP03A01.pdf |
IQ report |
Q:\2003-01-VP\10003\ICB-ICB02A02.pdf |
OQ protocol |
Q:\2003-01-VP\10003\OQP-OQP02A01.pdf |
OQ report |
Q:\2003-01-VP\10003\OQB-OQB02A02.pdf |
PQ protocol |
Q:\2003-01-VP\10003\PQP-PQP01A01.pdf |
PQ deficiencies list
The following deficiencies have been determined based on the checks.
| Aspect |
Description |
Measure |
|---|---|---|
not applicable |
not applicable |
not applicable |
Result of the performance qualification
The checks and activities have been carried out in accordance with the specifications of the qualification plan for the performance qualification.
The level of operational qualification is x complete. o not complete A list of deficiencies has been finalised. The facility is qualified and released for production. All changes to the facility are subject to the change control procedure (SOP A1000CC05). |
Summary As part of the preparation, measures must be established before commencing the actual packaging process to prevent confusion or mix-ups. The first stage of facility approval is line clearance after cleaning out the previous product. Once the facility has been set up, step by step, a first process sample is checked with regard to the specifications and the variable data so that production can be approved if the results are consistent. During operation, the process is constantly checked by test devices in the facility and repeated verification of the functionality of control devices by the personnel. This is supplemented by checks of packaged products. The function check and controls of packaged products, together with the approval of the facility for production, can be seen as in-process controls. The allocation of designated groups of people within the personnel is unit specific. Variable data, which is afixed during the packaging process, must meet requirements with regard to readability, accuracy and constancy. There are various techniques which can be used for this purpose. An important step at the end of a packaging process is the reconciliation of the packaging material. The significance of this process depends on the overall design of the facility and the process. |

