Packaging process
Here you will find answers to the following questions:
|
"Packaging shall mean the following processes: „All operations, including filling and labelling, which a bulk product has to undergo in order to become a finished product. Note: Sterile filling would not normally be regarded as part of packaging, the bulk product being the filled, but not finally packaged, primary containers." (See the glossary in the EC GMP Guideline.)
Packaging processes are generally carried out in packaging operation units, with the exception of on-line packaging. In these units, various bulk products are packed in primary and secondary packaging materials which frequently differ, e.g. if packed for different destination countries. Packaging processes carry a high risk due to their multiple effects on the product, for example.
A basic requirement of packaging is to minimise the risk of cross-contamination, cross-mixing and misidentifications (5.44 EC GMP Guideline). This will in principal require measures relating to both equipment construction and organisation. The constructional requirements for rooms, equipment and air conditioning are dealt with in the chapter on rooms (see chapter 3 Premises). The organisational aspect will be the major focus of the following text. The objective that was specified can only be achieved if all of these measures compliment each other, e.g. the more ideal the constructional preconditions, the simpler it will be to organise the processes. Older rooms and equipment located in the open air or which have very basic control equipment, for example, require greater differentiation in the process organisation and the control tests.
| Principles of GMP consistent packaging |
|
|---|---|
Constructional measures
|
Organisational measures
|
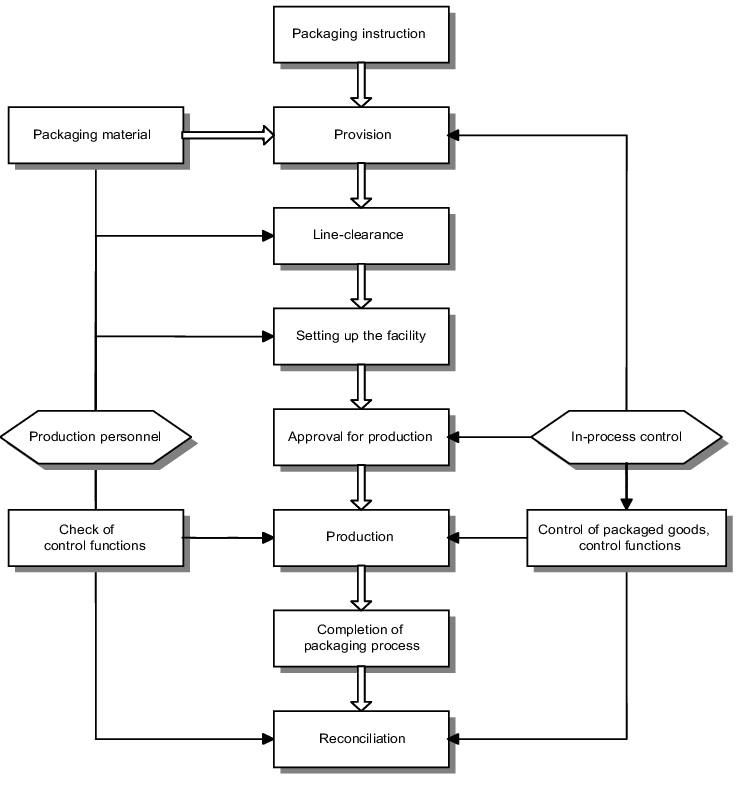
The basic sequence of a packaging process is set out in figure 13.B-2. It allocates the areas of production and in-process testing as groups of different functions, for example. Organisational variations on this are also plausible.
13.B.1 Allocation of packaging material
Packaging material may only be issued against a written order in specific quantities which correspond to the requirement specified, and by authorised staff. Packaging materials must be commissioned using the appropriate bill of materials from the packaging order. This bill of materials must be used to order types and quantities of materials in a logistical method. The order must be compiled by authorised staff in a defined area which is very close to the packaging area (both physically and in organisational terms). There should ideally be a segregation of the material flow of primary and secondary packaging materials, in order to minimise the risk to bulk goods from cartoning, for example. Depending on the cleanliness grade, different air locks should be passed through during transport. Personal hygiene measures, e.g. special clothing, washing hands will be required for this in addition to material conversions, e.g. changing from wooden pallets to pharma pallets (aluminium or plastic) and using an air syringe or compressed air to dedust. When the primary packaging material is not cleaned immediately prior to the packaging process there is a problem with the wrapping. Tubes, stoppers, rolls of foil, etc. are stored in the warehouse in their outer packaging. It must be guaranteed that this outer packaging is removed without dust and dirt contaminating the packaging material or the packaging equipment.
Possible ways to do this include various aspiration systems in the area where the materials will be converted or allocated. In addition, the double wrapping system can also be used. This involves, for example, placing a roll of foil in a polyethylene bag (e.g. in a drawstring bag for easy use). A second bag is needed to keep dust and dirt away during storage. These bags will then be removed in succession once the conversion is carried out. This then provides a basic protection.
It must be assured during the commissioning that the composition is clean to the order. A delivery of a mixture of different orders must not be accepted unless the materials are placed in interim storage before the actual commissioning and preparation. The preparation zone must be of a size adequate for its function and capacity, so that no mixups are caused. It may be advisable to use secured pallets in order to prevent misidentifications and unauthorised access. The materials could be stored in skeleton boxes or containers, for example, or in special pre-storage rooms for individual packaging rooms. The delivered goods must be inspected in all cases by packaging staff before they are placed in the packaging area. The inspection shall involve comparing the quantities and the identities of each container or roll against the bills of materials from the packaging materials orders. Loose, printed packaging material which is not in closed containers should be rejected for safety reasons.
13.B.2 Line clearance
| Equipment: VP-LC3 |
Batch packaging record |
Page x of y |
|||
|---|---|---|---|---|---|
| Version 03 |
Line clearance |
||||
| Material no.: MM03 |
Batch no.: AAA345 |
Order no.: kl33 |
|||
| yes |
no |
Date/ |
|||
Production status |
|
||||
Room |
|
||||
Product supply |
|
||||
Foils |
|
||||
Package insert |
|
||||
Folding cartons |
|
||||
Assembly lines |
|
||||
Conveyor balance |
|
||||
Bundling machine |
|
||||
In-process control samples |
|
||||
Comments |
|||||
Conclusion |
Line clearance completed |
||||
Line ready for setting up |
|||||
"Before packaging operations are begun, steps should be taken to ensure that the work area, packaging lines, printing machines and other equipment are clean and free from any products, materials or documents previously used, if these are not required for the current operation. The line-clearance should be performed according to an appropriate check-list." (5.45 EC GMP Guideline, see also figure 13.B-3.)
An important element in preventing cross-contaminations, cross-mixing and misidentifications in the field of packaging is the continuous cleaning of the packaging line. In addition to bulk-specific cleaning in accordance with valid cleaning procedures, this also involves removing loose packaging materials from the previous operation in order to prevent bulk products from being transferred to subsequent products or batches and the wrong package inserts or the wrong labels from being adhered.
The procedure must be defined with precision. A definition of when and to what extent the operation will be carried out should be given. These equipment-related specifications must be formulated clearly to exclude the possibility of staff interpreting them incorrectly. A checklist can be used to describe the critical equipment-specific areas and enquire into their review. Product transfers can only be excluded if these work instructions are followed accurately. Both the machine itself and also its surroundings and/or the room where all utensils, residues and containers are stored must be appraised after the operation has been carried out. Samples which have been removed from the packaging process must also be systematically removed. The process description must specify who is responsible for which cleaning stage and for which control tests. The production staff can be responsible for cleaning and dismantling, persons responsible for the in-process controls (also frequently referred to as line investigators) can be responsible for controls and release, by means of example. The completed checklist is enclosed in the batch documentation of the following product as evidence that those activities have been completed. The line clearance following assembly of the machines for a new packaging order shall be described separately. See chapter 13.B.5 Release for production.
13.B.3 Labelling
The packaging lines must be labelled with regard to their current status. This involves affixing information in a way that it is clearly visible. This gives the staff a permanent opportunity to compare data relating to the order with the materials that were received.
| Labelling of packaging equipment |
|---|
|
13.B.4 Control functions
The possibilities for machine-assisted monitoring systems are constantly increased by technical development. An increase in the speed of the production process leads to a rise in the requirements placed on the technical control functions. These functions can guarantee the quality of the final product. A series of optical-electronic control elements such as fill controls, control balances, code readers, thermometers, counter units, various inspection systems etc. are used. These make use of regulation systems (e. g. thermometers), data collecting systems (counters) and also sorting functions (control balances with emission systems).
The control functions must be qualified and calibrated when they are used and their functionality for critical elements during the production process must be checked (see chapter 13.B.6 In-process controls). It must be guaranteed that during the production process it is not possible to switch off the controls or to operate without these facilities. Malfunctions must always be thoroughly investigated. If it is necessary to operate the equipment without a control function, e.g. if a code reader fails for a short period of time, this deviation must be evaluated in a risk evaluation and the measures taken, e.g. additional checks or supervisory personnel must be authorised by the responsible persons. Naturally, details must also be entered in the log book. Examples of content controls, code readers and inspection systems are given below.
Fill controls
Fill controls play an important role in GMP and productivity. They are used to monitor the correct filling of the primary container with blister wells for example, during the current process. Depending on the technical equipment, presence testing (bulk available?) and also qualitative evaluations (is the bulk within the tolerances?) can be carried out.
These optical-electronic systems are checked as part of the qualification and the production preparation with regard to the functionality with packaging materials and the bulk products. Different parameters can be monitored. For blister lines, for example, standard double fillings, location errors, fragments or mends are normally detected (by a black and white system).
| General aspects |
|
|---|---|
Light quality |
|
Transmitted light |
|
Reflected light |
|
Transmitted light/reflected light combination |
|
Foil quality |
|
Dye, transparency |
|
Products |
|
Dye, transparency |
|
Breakage tolerances, missing products, characteristics |
|
Spalling on surfaces, stains |
|
Sensors |
|
Dissolution, reading speed |
|
System |
|
Calibrational capacity, reproducibility |
|
More complex systems (colour systems) also make it possible to monitor colour deviations (e.g. differential measurement of colour type, saturation, intensity) and geometric parameters (differential measurements of surfaces and shape differences (circle/hexagon). Error tolerances must be defined. Formats which have been tried and tested should ideally be reproduced. This can then reduce waste and also the time required. This reduction can be achieved using a knowledge storage system for basic data, e.g. blister segmentation through various well diameters and also through product-related data. Packaging for solid oral dosage forms should illustrate important elements of the requirements to be met by the content controls (figure 13.B-5).
Code reader
Code readers are used at various stages of the packaging process. The object of the checks is packaging materials with a high risk of misidentification leading to a high risk for the consumer. These include the package inserts, the folding cartons and the labels. Different types of bar codes are used. The tendency is towards smaller and smaller codes, e.g. 3D barcodes which have proven to be very safe due to their high redundancy. Systems for monitoring coloured ampoule rings or flip-off coloured caps can also be included in the above.
Inspection systems
The function of the inspection systems is to select defective products before and during the packaging process. They include inspection systems for detecting suspended solids in solutions, e.g. in injection fluids and liquids. This apparatus normally consists of a sensory unit (e.g. a video camera), a computer unit with data saver and a monitor. Cracks in glass, filling levels and other deficiencies can also be detected. In a wider context, the content controls in the sense of bulk product monitoring can also be included. Strict requirements are made of the qualificational capacity for such systems.
13.B.5 Release for production
The facilities are released for production of the relevant packaging order once an inspection has been carried out to ensure conformity with technical production requirements and with the order requirements. The exact sequence with all important items must be described in detail. The items checked must be recorded. Similarly to the line clearance, it is also advisable to determine the procedures to be followed using a checklist (figure 13.B-6).
Repeat checks on the readable, batch-related imprints enables the controls to be conducted with the data specified in the packaging instructions. Errors such as transposed digits should be avoided as much as possible by writing down the actual and target values directly underneath each other
Initial sample controls are normally performed after equipment has been assembled, meaning that initial samples are checked from each order by in-process controls staff. This includes intensive checks on the goods packed (imprint, layout, batch description, expiration date, additional texts, appearance of the packed bulk products and check dimensions, blister tightness, etc.).
If values do not conform with specifications remedial action can be undertaken on the equipment where permissible or possible. This will then be documented in the batch packaging record. The release from production must be documented in a manner that is clear and visible (figure 13.B-7).
The samples produced when the equipment was set up should be removed before production gets underway and be separated as rejected goods in the same way as during the production process.
It is advisable to have the release for production carried out by a group of people who do not interact with production staff. This group can be made up of staff who conduct the in-process controls depending on the type of organisational system (see chapter 13.B.6 In-process controls).
| Equipment: PL-LC3 |
Batch packaging record |
Page x of y |
|||
|---|---|---|---|---|---|
| Version 034 |
Assembly record |
||||
| Material no.: MM03 |
Batch no.: AAA345 |
Order no.: kl33 |
|||
| yes |
no |
date/signature |
|||
Production status |
|
||||
Cleaning |
|
||||
Tools |
|
||||
|
|||||
Shaping tools |
|
||||
Bulk product feed |
|
||||
Level controls |
|
||||
Content control |
|
||||
Sealing station |
|
||||
Expiration date |
Batch No. |
||||
1st blister 2nd blister 3rd blister 4th blister |
|||||
Manufacturing instructions specifications |
|||||
|
|||||
Punch |
|
||||
Lowering device |
|
||||
Blister sorting device |
|
||||
Exhaust station |
|
||||
Congestion controls on the transfer line |
|
||||
Congestion controls on the stacking shaft |
|
||||
Adhesive point controls |
|
||||
Stacking shaft |
|
||||
Brochure |
|
||||
Folding cartons |
|
||||
Expiration date |
Bat.des. |
||||
Folding cartons Manufacturing instructions specifications |
|||||
|
|||||
Code reading |
|
||||
Transfer to control balance |
|
||||
Control balance |
|
||||
Generalities |
|
||||
Comments: |
|||||
Conclusion |
|
||||
| Equipment: VPP01 |
Batch packaging record |
||||
|---|---|---|---|---|---|
| valid from 14th July 2003 |
Line release by line inspectors |
||||
| Material no.: Batch no.: AAA345 |
Order no.: |
Page x of y |
|||
| Type of inspection |
corresponds to |
Date/ |
|||
| yes |
|||||
The line identification labelling complies with the instruction (SOP 7050). |
|||||
Line clearance has been completed and documented. |
|||||
Material number and batch number of bulk products correspond to the commissioning order. |
|||||
Material number and batch number of the foils correspond to the commissioning order. |
|||||
Content controls: Blisters with missing compressions are ejected (SOP 7053). |
|||||
The blisters are undamaged (SOP 7055). |
|||||
The blisters are free from deformation (SOP 7055). |
|||||
Ejection counter controls: all blisters in a timing cycle are discarded (machine type I). |
|||||
Blisters are complete, undamaged and imprinted, the number of tablets complies with the manufacturing instructions (SOP 7056). |
|||||
The tightness of the blisters in one cycle complies with (SOP 7057). |
|||||
Variable data on blisters complies with the packaging instructions. |
|||||
Perforation: Blisters are free from damage and can easily be peeled away from the perforated seam (SOP 7059). |
not applicable |
||||
Codes on package inserts and folding cartons comply with the manufacturing instructions. |
|||||
Code reader for the package inserts functioning correctly (SOP 7060). |
|||||
Luminescence control functioning correctly |
|||||
Code reader for the folding cartons functions correctly (SOP 7062). |
|||||
Conveyor balance functions correctly (SOP 7065). |
|||||
Variable data on the folding cartons ( if available, on labels) comply with the batch processing record. |
|||||
Finished packs are complete and undamaged (SOP 7070). |
|||||
The number of folding cartons per bundle is correct, the folding cartons are undamaged (SOP 7071). |
not applicable |
||||
Vignettes are placed in the correct spot (according to the packaging instructions). |
not applicable |
||||
Material numbers and batch numbers of all secondary packaging materials comply with the order. |
|||||
Samples of printed packaging materials were removed (SOP 7080). |
|||||
Release of packaging line |
|||||
Release of packaging line |
Time: |
||||
13.B.6 In-process controls
| Minimum requirements for in-process control tests on equipment during packaging |
|---|
|
Different control levels are responsible for guaranteeing quality during the packaging process. Facility-specific control elements ensure a proper regulation of the manufacturing process (see chapter 13.B.4 Control functions). The functioning of these control elements must be checked regularly during the packaging process (see chapter 13.B.6.2 Function inspections). In addition, the primary packaged goods and the finished medicinal products are also checked. All checks are carried out in accordance with the specifications in the packaging instructions. They are documented in the record with their results. This data is then taken into account for the final approval.
| Controls to guarantee quality |
|---|
|
The different control tests are normally conducted in a time-regulated manner, that is, during the continuous packaging process, control tests are carried out and/or samples taken at regular time intervals. Exact specifications for the frequency, the type of documentation and the responsibilty for the individual tests must be drawn up as part of the process organisation. When defining the frequencies, be certain to specify tolerances, e.g. every 120 minutes ± 15 minutes. This enables the equipment to be operated continuously and the staff to be deployed in an uninterrupted manner (figure 13.B-10).
| Equipment: PL-LI05 |
Batch packaging record |
|||||||
|---|---|---|---|---|---|---|---|---|
| valid from 22. 12. 2003 |
In-process controls: Line inspectors |
|||||||
| Material no.: MM03 |
Batch no.: |
Order number:kl33 |
Page |
|||||
| Test interval: 60 min ±15 min: |
||||||||
| Date |
||||||||
| Time |
||||||||
| Test items |
corresponds to |
corresponds to |
corresponds to |
corresponds to |
corresponds to |
corresponds to |
corresponds to |
corresponds to |
Vignette positioned correctly |
||||||||
Perforation correct (SOP 7059) |
||||||||
Bundling correct |
||||||||
Dispatch label positioned correctly, variable data correct |
||||||||
Variable data on FS correct and legible |
||||||||
PBL available and undamaged |
||||||||
Blister undamaged |
||||||||
variable data on blisters correct and legible |
||||||||
Foil run correct |
||||||||
Blister filling correct |
||||||||
Well design correct |
||||||||
Folding carton code reader function correct* |
- |
- |
- |
- |
||||
Package insert code reader function correct* |
- |
- |
- |
- |
||||
Luminescence controls correct* |
- |
- |
- |
- |
||||
Deformation ejection controls correct* |
- |
- |
- |
- |
||||
Conveyor balance function correct * |
- |
- |
- |
- |
||||
Bundling machine function correct* |
- |
- |
- |
- |
||||
Blister impermeability complies with* (SOP 7057) |
- |
- |
- |
- |
||||
Checked by/signature |
||||||||
Comments: |
* Control tests every 120 minutes |
|||||||
13.B.6.1 Organisation
Various organisational formats can be found for in-process controls within the packaging process. One possibility is the distribution of functions and responsibilities into different groups. This includes function tests and standard tests which are carried out by the production staff. A start-up control, that is, a detailed check of the first goods produced for the order and culminating in line release, is conducted by a group which reports directly to either the head of quality control or at least the head of section, to ensure that an independent appraisal is achieved, In addition, the groups also conduct control tests throughout the packaging process as well as function tests. Depending on the company's terminology, these groups are also referred to as line inspectors or monitoring staff.
The responsibilities for the various control tests must be clearly specified in all cases to prevent misunderstandings from occurring.
| Responsibilities during in-process controls |
|---|
Production staff
|
Line investigators
|
13.B.6.2 Function inspections
The inspection functions which are used must be inspected regularly for their operability. The intervals between inspections must be defined in equipment- and product-related terms. In addition to maintenance factors and calibration checks, the frequency of the inspections must be specified in the packaging instructions and documented accordingly.
The example of the cartoning machine (after tubes have been filled) illustrates the relationship between control elements, control tests and quality attributes (figure 13.B-12).
| Quality attributes |
Quality-related |
Quality controls |
Analyses |
|---|---|---|---|
Completeness of packages |
|
|
|
Accuracy of the packaging material |
|
|
|
Package closures |
|
|
|
Condition of package (undamaged, clean) |
|
||
Correct data imprints |
|
|
Ideally, simple and pragmatic solutions are devised for the function tests to be carried out. A function test for code readers can be conducted using an identified folding carton or package insert (e.g. by changing the bar code by adding a further bar using a felt-tip pen). The code reader must display a failure message in the display panel. In addition to the code reader, this also checks the functionality of the ejection equipment. The ejection controls can be tested by removing the test item before it is ejected. The test item should be marked in a way that makes it easy to recognise, to prevent it from becoming accidentally mixed up. The marking can be carried out by colouring the edges, so that the supervisor will notice it in the feed.
Inspections on conveyor balances in process can be carried out using identified packages whose weights have been specially reduced. Inspections to counteract overfilling can be unnecessary with regard to medicinal product safety (except for: filling pressure), but can uncover function errors during the filling process. One possible way to do this is to remove the package insert, for example, or to reduce the fill level (e.g. by removing one effervescent tablet from the tube). The main object of this inspection is the functioning of the balance in addition to the interaction of the ejector function with the balance.
"Products which have been involved in an unusual event should only be reintroduced into the process after special inspection, investigation and approval by authorised personnel. [...]" (5.55 EC GMP Guideline). Packaging materials which are removed during control tests must therefore not be added to the regular goods except in cases where the control test unit has made an error. It does, however, remain critical and must be documented with "[...] detailed records [...]". To guarantee that they are not placed back into the process, collection vessels which do not allow manual access to the materials can be used. This vessel will then not be opened until the reconciliation process takes place. It is also advisable not to add goods removed during in-process controls straight to the goods production. Attention should also be paid to pre-product samples which were taken during in-process controls and which are removed during the line clearance inspection. This is especially important for partially-packed goods such as ampoules, tubes or blisters still being produced.
13.B.6.3 Checking (partially) packed goods
Packed or partially packed goods can be checked while the equipment is running. In addition to medicinal dosage-related checks, e.g. leak tests on blisters, AQL lists are used in parallel to the packaging materials checks (see chapter 13.A.5.3 Defect evaluation lists). The number of random samples corresponding to the size of the order is removed from the lists. The types of defects are then defined in medicinal product terms. A defect classification system for effervescent tablets (filled into tubes) can be found below by means of an example (figure 13.B-13).
| Error class |
Error type |
|---|---|
over-critical |
|
critical |
|
Major error |
|
Minor error |
|
These classifications must be imposed in accordance with company specifications for each dosage form. They must be oriented towards both the legal specifications and the company quality agreements, that is, the legal minimum requirements must be considered as the absolute minimum threshold. Many of the errors found during visual inspections are very much a question of interpretation. The difference between not legible and moderately legible, for example, depends on the individual interpretation of the checker. In order to make the evaluation of different features and the degree of personal interpretation as objective as possible, it is advisable to list examples for the different categories - if possible of errors that are only just acceptable and errors that are not acceptable. This can easily be done by compiling a library of photographs, Using digital photographs enables these records to be used in a cross-functional manner (when checking different packaging lines or plants and also for standard cross-location training purposes).
13.B.7 Cleaning primary containers
"Containers for filling should be clean before filling. Attention should be given to avoiding and removing any contaminants such as glass fragments and metal particles." (5.48 EC GMP Guideline). The cleaning of bottles for non-sterile liquids, for example, will follow a procedure which is identical to the cleaning of other product contact surfaces. If dust is the possible source of contamination, for example, the container is pre-flushed with demineralised water with optional ultrasound assistance. After the container has been flushed again, it is dried using heated compressed air. The cleaning operations are normally connected with the filling and/or packaging operation on the assembly line, meaning that interim storage, which carries the risk of recontamination, will not be necessary.
13.B.8 Labelling
"Normally, filling and sealing should be followed as quickly as possible by labelling. If it is not the case, appropriate procedures should be applied to ensure that no mix-ups or mislabelling can occur." (5.49 EC GMP Guideline). It is evident that all unlabelled containers must be temporarily stored in sealed containers. Great care must be taken when labelling these containers. It should ideally be possible to simply determine the number of containers, so that the reconciliation process for labelling will carry weight. Before the containers are introduced into the labelling process, a visual inspection of the identity of the packed containers as well as the identification on them is conducted.
"Special care should be taken when using cut-labels and when over-printing is carried out off-line. Roll-feed labels are normally preferable to cut-labels, in helping to avoid mix-ups." (5.51 EC GMP Guideline). Cut-labels should always be considered to carry an increased risk. Containers with cut-labels must be stored and transported in sealed containers. Preferably, roll labels should be used whenever possible.
In special cases, vignettes containing pricing information for specific countries, for example, can be affixed to the containers. Some of these vignettes (e.g. bollini) are continuously numbered and can be affixed to individual folding cartons. The individual vignettes are downloaded and assigned by software to the relevant bundle prior to banding.
Once the product has been bundled, a bundle label containing the information from the appropriate vignette is then added. It should also be ensured that unlabelled packages or cut-labels are removed, or at least that they are not fed into the undamaged goods section in the event of machine stoppage. Depending on the degree of automatisation, measures such as emptying the line in accordance with a checklist or opto-electronics sensors with ejection devices can be found.
13.B.9 Variable data
The batch description and expiration date are referred to as variable data. This data is normally added during the packaging process as a secondary imprint to the corresponding packaging material (figure 13.B-14). Further company-related information which sometimes needs to be added in a second imprint are pricing details, doctors' sample details and warnings. Particular caution should be exercised when handling printed materials during a pre-printing operation (chapter 13.A.5.4 Storage). Variable data must satisfy the following requirements with regard to legibility, accuracy and durability:
Legibility
Embossing products without using colour is not an ideal solution. Embossing without colour is still carried out for blister strips and tubes. The use of colour is a better alternative for folding cartons. The data must be added in very legible font, meaning that a person with normal vision can read them without a visual aid. The font characters must be of a sufficient size and must offer a sharp contrast to the background colour. A font size of 2 mm (size 8 font) is considered adequate.
| Identification techniques |
|
|---|---|
Stamping equipment |
|
Embossing units |
|
non-contact systems |
|
Accuracy
The accuracy of the data must be checked before and during the production process. These checks are recorded during the in-process controls. Visual inspections assume great importance when checking identification added to a product. Mechanical detection of product identification is only practical for identification purposes when adding OCR fonts or code symbols (using a laser printer). The read-only result is compared with the reference symbol (entered for batches or at the beginning of production). If the two results concur, the item (e.g. label) is released. Different formats or fonts should ideally be used in a studious manner (figure 13.B-15).
| Typical functions of plain writing control systems |
|
|---|---|
Controlling of |
|
Test items |
|
Detection of mistakes in symbols |
|
Durability
The labelling is acceptable if it is not easy to wipe off, illegible, or easy to remove or change. This normally means that it must be printed.Self-adhesive labels may only be used if they can only be removed when damaging the background surface.
13.B.10 Imprints
In addition to variable data, complete imprints of printing blocks are possible as well as complete digital printing.In this case, the advantage is, that only unprinted packaging materials is needed.The storage space used for the materials will decrease provided that the raw foils can be used for the majority of the product.This means that less obsolete packaging materials will need to be destroyed and less waste will appear (production on demand).The time taken to equip and convert the equipment for on-line designs can be reduced (print and apply technologies).
| Aspects of printed identification |
|---|
|
The printing devices must satisfy the product requirements, e.g. package inserts, meaning that they must be comparable with those for the packaging materials supplier.It must be guaranteed during the qualification process that a code number (e.g. order number and material number) are assigned faultlessly.If digital printing systems are used, that is the print data and images generated from the file directly particular requirements are made of the qualification such as securing the sequences and data paths. It must be clearly specified who issues the print release and at what time, as well as the usability of the printout.
13.B.11 Reconciliation
| Equipment: PLBIL03 |
Batch packaging record |
||||||
|---|---|---|---|---|---|---|---|
| valid from 14th July 2004 |
Reconciliation of packaging materials |
||||||
| Material number: MM03 |
Batch no.: |
Order no.: kl33 |
Page x of y |
||||
Yield |
|||||||
Package yields |
Number of full cartons/contents (e.g. 40 per 360 PG) |
Number of packages in overflow carton e.g. 1 to 39 PG) |
Date/ |
||||
Detection of waste during packaging |
Folding cartons |
User information |
Aluminium foil |
Labels |
Date/ |
||
Tally sheet |
- |
||||||
Total quantity |
- |
||||||
Packaging material |
Supply quantity |
Consumption |
Waste |
Redelivered quantity |
Yield |
Date/ |
|
Folding cartons |
|||||||
User information |
|||||||
Aluminium foil |
|||||||
Brochures |
- |
- |
- |
- |
- |
- |
- |
Labels |
- |
- |
- |
- |
- |
- |
- |
printedtubes, etc. |
- |
- |
- |
- |
- |
- |
- |
Dispatch labels |
- |
- |
- |
- |
- |
- |
|
* Package yields, + total quantity of samples |
|||||||
Deviations outside of tolerance must be justified: |
|||||||
up to 5, 000 packaging units 5 ,001-100,000 packaging units from 100,001 packaging units |
85-115 % 92.5-107.5 % 95-105 % |
||||||
Comments: |
|||||||
The results of the reconciliation comply with the specifications (SOP 7089) |
o yes |
Date/ |
|||||
"[...] the quantities and reference number or identification of all printed packaging materials and bulk product issued, used, destroyed or returned to stock and the quantities of obtained product, in order to provide for an adequate reconciliation." (4.18i EC GMP Guideline). As carried out during the production stages of bulk goods, checks on the yield must be conducted to ensure that it is within the required tolerances. For packaging, the additional materials supplied are also reconciled, so the number of transfer errors can be estimated. The requirement that the exact number of package components be known can only be met with great difficulty using the declared quantity as a basis, as under- and over-delivered quantities from the packaging materials supplier are frequently discovered. The weighing of paper or cardboard products is a basic guide which, depending on the degree of treatment during storage is assigned an error tolerance of at least 1 % . The use of counters for labels and package inserts, for example, offers the possibility to actually compare the counters used with the yields. The results of this comparison must be set out in the batch documentation. For rolled and bundled goods, a reconciliation of the packaging materials only offers a basic guide for a retrospective evaluation of the packaging process. The possibility of detecting the exact amount using a rolling process with a counting unit presents a critical step which can lead to a change in the subsequent rolling process. Piece goods, such as bottles, can, on the other hand, be easily reconciled. The yields (in %) must be provided with approved tolerances (e.g. 5 %). Deviations (e.g. in this case > 5 %) must be justified in an error evaluation process. The importance of reconciliation is mainly set against the backdrop of existing factors (room quality, equipment design, material routes, staff routes, staff qualifications) in order to minimise the risk of cross-mixing and misidentification.
13.B.12 Safety features
To protect against falsified medicinal products, organisational factors must be taken into account in addition to the possibilities offered by packaging material technologies (see chapter 13.A.4 Protection against counterfeit medicinal products). In addition to the customary risks such as regulated access to approved or rejected or obsolete bulk products, inspected and documented disposal of production waste and the corresponding reconciliation are also important. The pharmaceutical company is responsible for ascertaining that the waste disposal company disposes of the product properly and professionally, by carrying out a qualification of the waste disposal company, for example.
13.B.13 Completion of a packaging process
Once a packaging process has been completed, the batch-related packaging materials (e.g. labels) are destroyed following reconciliation. This must be recorded. Non batch-related printed packaging materials (see chapter 13.A.5.4 Storage) can be redelivered to the warehouse or to the packaging materials store. These processes must be set out clearly in an operating procedure to enable the packaging material that is to be destroyed or redelivered to be assigned accordingly in a manner which is clear for the appropriate staff. In addition the procedures for redelivery with the identification, packing and declared quantities elements must be specified in a system.