Freeze drying
Here you will find answers to the following questions:
|
The process of freeze drying can be used to very gently obtain dry substances from aqueous solutions. Freeze drying is primarily used to preserve the stability of sensitive substances such as hormones, antibiotics, enyzmes, protein preparations, blood preparations, serum, vaccines, etc. Many medicinal substances decompose in water over time. In order to keep them sterile and free of particles, they are dissolved and sterile-filtered. Containers filled under aseptic conditions are subjected to a freeze drying procedure, whereby the water is extracted (dried) from the frozen solution. This results in a stable, sterile powder or solid "cake". The containers are then aseptically sealed, for example ampoules undergo tip sealing at the sealing station of an ampoule filling machine, or injection vials are sealed with freeze-drying stoppers in the freeze dryer. The resulting readily soluble powder (lyophilisate) is not dissolved in sterilised water for injection until immediately before the medicinal product is used. The solution formed can then be injected.
12.J.1 Description of the procedure
The characteristic feature of freeze drying is that the water is removed from a frozen drug product without defrosting, i.e. through sublimation.
The usual liquid/gaseous phase transition that takes place in a conventional drying process is replaced by the phase transitions liquid/solid (freezing) and solid/gaseous (otherwise known as sublimation or freeze drying).
After freeze drying, the original properties of a drug product are retained in an optimal way. Freeze drying is the method of choice for the preservation of sensitive substances, particularly if they are of biological origin.
Execution
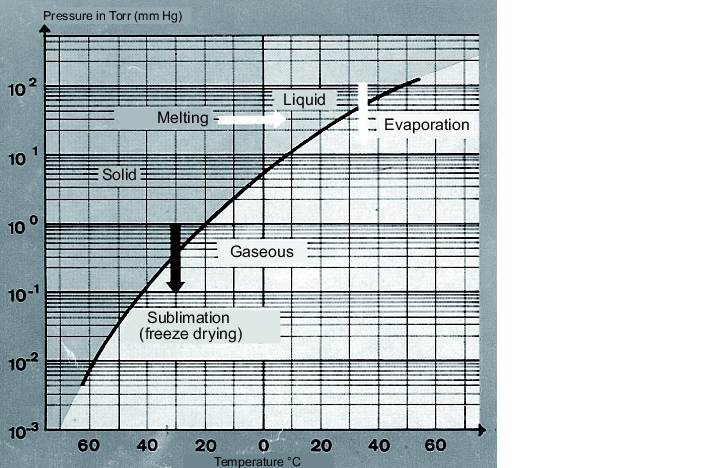
First, the solution is frozen in the container (ampoule, bottle) and is then subjected to a vacuum, taking care to ensure that no defrosting occurs. In the vacuum, steam molecules escape from the frozen, aqueous substance until a partial pressure of steam corresponding to its temperature (steam saturation pressure) develops above the frozen substance (see figure 12.J-1).
For drying, appropriate pumps (vacuum pumps) are used to constantly extract condensable gases and steam using the "steam pump" (condenser). Steam molecules escape from the frozen product sublimation until the product is dry (minimum residual water content). After freeze drying, the ampoules must therefore be sealed in a room under adjusted dry conditions (< 20% rel. humidity). Bottles enter the freeze dryer with a special freeze-drying stopper already in place, and following drying, are sealed in the facility by pressing in the stopper.
The method of freezing and the cooling temperature are determined by the properties of the product, the size and shape of the containers, and the most important factor of the eutectic temperature (solidification temperature of solutions). This temperature also affects the subsequent appearance of the "cake".
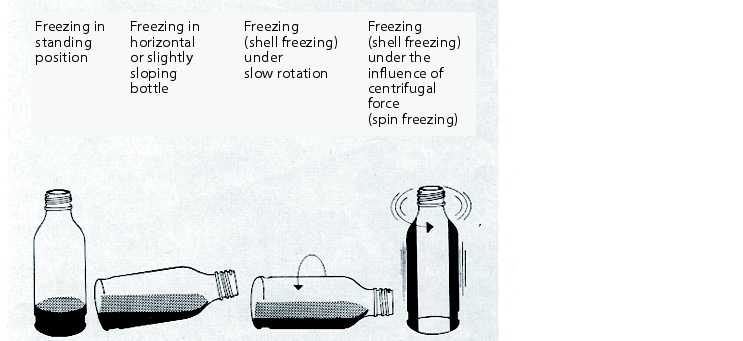
The freezing temperature must be adjusted to suit the product - for example, it can be -15 °C for one product or -50 °C for another product. Systems should be specifically selected for this reason, or the freezing process should be performed outside the freeze drying system in special freezing facilities (such as a rotation method). Different methods of freezing are shown in figure 12.J-2.
Layers of the drug product should not be higher than 2 cm, in order not to inhibit the transport of steam through the product. The ideal temperature range for freeze drying is -20 °C to -40 °C, since small ice crystals are formed in this range, and hence the steam is quickly transported through the drying product during the drying process. It should be noticed that at low substance concentrations no "cakes" of uniform appearance can be formed in the solution to be dried. For caking to be achieved, the following measures are possible:
- Add "additives" such as dextrin to form a matrix on which the drug substance can be evenly distributed. This point has to be considered at the development stage of the drug product.
- Increase the concentration of solid matter content, so that the "cake" is not too severely deformed on drying (breakages, clumping), and to ensure it has a uniform appearance after drying.
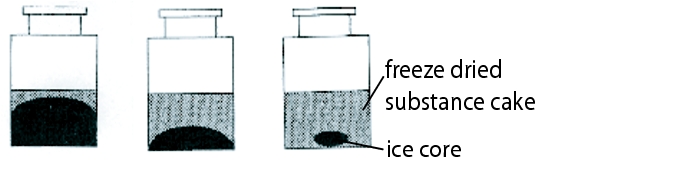
- Select the container according to the ratio of wet surface/volume of solution/solid matter content. This is also a task for the development phase of the drug product (figure 12.J-3).
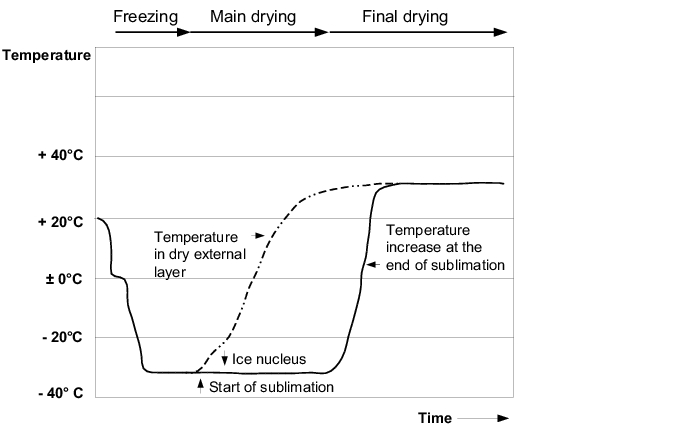
The duration of freeze drying depends on the temperature in the ice-containing part of the drug product - the higher this temperature, the faster the process. It is therefore of utmost importance to control the temperature in the ice-containing part of the drug product to be dried. The ice nucleus of the drug product must be prevented from thawing during heating at all costs. Monitoring the electrical conductance in the frozen product is used to help regulate the system. When the frozen solution is warmed up, there is a sharp decrease in electrical resistance when the eutectic temperature is reached, which is interpreted as a sign that the ice in the drug product is beginning to melt. The measurement of electrical resistance can therefore provide information about the behaviour of the drug product during the freezing and drying process. The drying process is described in figure 12.J-4.
12.J.1.1 System components
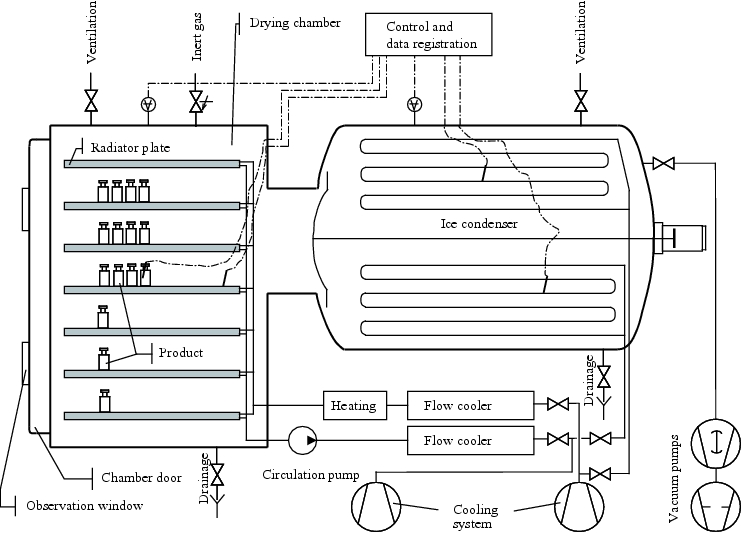
Freeze-drying chamber
The freeze-drying chamber is where the drug product is placed, and consists of trays that can be cooled or heated. Multiple trays can be aligned, for example, over each other, and can be moved together using a hydraulic mechanism to close bottles with freeze-drying stoppers. Freeze dryers are supplied by many manufacturers in a wide variety of designs to accommodate the diversity of products and containers used figure 12.J-5).
The cooling and heating system, using silicon oil as a heat transfer medium, must enable even changes of temperature across the trays and continuous temperature regulation. The low temperature level of the trays during freezing is determined by the requirements of the drug product. The distance between the trays depends on the size of the containers for the drug product. The total tray surface area, the maximum loading with containers and the maximum thickness of layer of the solution to be dried result in the basic capacity for the amount of ice collecting in the steam pump (condenser).
Steam pump (condenser, cryopump)
The action of the steam pump is based on a surface area of very low temperature (lower than the temperature in the drug product) on which the steam condenses and becomes ice, and thus occupies a very small space compared to its gas volume. The steam, which, according to gas laws, will spread to occupy the entire remaining space (chamber and condenser), is then extracted from the chamber space. This means that on condensation, the gas proportion becomes reduced, and the gas is transported from the chamber to the condenser. This is only possible, however, if obstacles caused by molecule collisions with water molecules are prevented, by the extraction of non-condensable gases by vacuum pumps in the initial phase.
The construction of the condenser is a very important factor for its effectiveness. Its lowest possible end temperature determines the partial pressure of steam that the "steam pump" can achieve. The intermediate valve of the chamber/condenser transition must have a large transition diameter, and must be designed to deal with the large volumes of gas. At minus 40 °C, ten kilograms of ice represents a gas volume of approx. 100,000 m3, which has to pass through the narrow transition to the condenser within a few hours. The condensation heat produced during condensation is equivalent to the amount of heat passed to the frozen drug product during freeze drying by the heating of the trays to compensate for the sublimation coldness.
Since most drug products have a very low residual moisture, the end temperature of the ice condenser must therefore be particularly low (see figure 12.J-1).
To pump the last remaining steam (usually a very small amount) from the drug product, vacuum pumps are used that have an end vacuum of up to approx. 10-5 mbar. The interaction between the steam pump and the vacuum pumps for pressure regulation is controlled by programmable automatic programs. It must also be possible to control the freeze dryer manually, although this is not ideal as it consumes energy and time, and is personnel-intensive, since control points must be monitored every two hours.
Ideally, the pressure in the drying chamber should be set at a level so that the heat transfer from convection caused by the molecules in the drying chamber contributes significantly to heat transport. When selecting a system, it is also important to consider the maximum condenser capacity (condenser capacity per 24 h, or total ice capacity before thawing).
Measurements
For the optimum freeze drying process for a particular drug product, you need the following information:
- The required temperature in the ice nucleus of the drug product
- The maximum temperature in the layer that is already dry
- The approximate residual moisture at which the drying process is considered complete (taking into account the desorption isotherm of the drug product)
- Pressure measurement (chamber/condenser).
This results in the following measuring elements:
- Heat sensors
- At least two product sensors (thermocouples)
- Condenser temperature sensor (Pt 100)
- Sterilisation temperature sensor (Pt 100)
- Tray temperature sensor (Pt 100)
- Conductivity measuring point
- Pressure measuring equipment
- Vacuum measuring tube (principle of heat transfer, available from several manufacturers)
12.J.2 Qualification of a freeze dryer
The use of a freeze dryer must guarantee that any aseptically filled objects handled in the system achieve the required result - namely the gentle removal of water content (except for a defined permitted residual moisture) - without microbiological contamination. This requirement is ensured through the technical design and qualification.
The following points need to be considered in the qualification of a freeze dryer:
- Materials
- Pipe connections
- Electrical components
- MSR components
- Function
- Monitoring
- Documentation
- Technical sequence of loading and unloading
- Transport from the filling machine to the freeze dryer in accordance with the cleanliness grade
- Uniform temperature transmission and thus freeze drying conditions throughout the contact areas of container/transport vessel/tray.
12.J.2.1 Installation qualification (IQ)
The execution of the tests is described in detail to ensure that the same tests can be accurately replicated in the future. Measured values must be recorded in the test record and documents (printouts, diagrams, etc.) must be included as attachments. Figure 12.J-6 shows an example of some test points.
| Tests in installation qualification |
|---|
|
Calibration of measuring points
The scope of the IQ includes defining measuring points, the type of measurement, operating range, adjustment points, calibration intervals and levels of accuracy. The quality-relevant measuring points of freeze dryers are as follows:
- Temperature (condenser, trays, product)
- Conductivity
- Time
- Pressure (vacuum, excess atmospheric pressure)
Measured calibration values must be compared with reference systems from a higher class. If measured values are outside the acceptable measured value tolerance, the measurement chain must be adjusted and recalibrated (see chapter 4.G Calibration). The operational qualification cannot take place until a successful initial calibration has been performed.
Heat sensors
Pressure and vacuum resistant control heat sensors are used, which must be suitable for use in the fine vacuum phase and resistant to steam sterilisation pressures. The feeds are connected to a measuring point recorder of the appropriate quality grade. The thermocouples should be calibrated at 0 °C.
Vacuum measurement/pressure
Measuring tubes for fine vacuum measurement in the range 10-3 mbar (hPa) according to the principle of heat transfer are recommended by manufacturers for pressure regulation in different variations and measuring ranges. These can be used to indirectly determine the product temperature according to the steam-pressure curve over ice. The calibration should be performed in several steps from <10-4 mbar to 1000 mbar. The excess pressure in the sterilisation phase (which should also be considered as a cleaning step) with super-heated saturated steam is measured using pressure transmitters, which are tested against a control device at pressures of 1.1 and 2.1 bar (abs). As this is a process for sterilising surfaces that do not come into contact with the product, the temperature should only be measured in a few points (coldest spot - condense water drainage, chamber condenser).
12.J.2.2 Operational qualification (OQ)
In the operational qualification, all the operating states of the freeze dryer are simulated to their limits (worst case), and the results are documented. Critical test points are listed in figure 12.J-7.
| Test points in operational qualification (extract) |
|---|
|
The test to check the uniform heat distribution on the plates and heat transfer to the objects is particularly important for the formation and evenness of the "cakes" in all containers. This test also proves that all objects are subject to the same temperatures in the freezing and drying phases. Suitable substances for test runs are, for example, dextran or mannitol (5 %) (figure 12.J-8).
| Function tests |
|
|---|---|
Recorder feed of the external device |
|
Check that the freeze drying time measurement is functioning correctly. |
|
0 °C comparison |
|
Tightness test |
|
Media quality
During qualification, the quality of the pure steam and the nitrogen used to ventilate the freeze dryer is also tested. The warm potable water used for thawing the condenser is also analysed. The data from these investigations can be used, as long as current values are available from monitoring or are determined during process validation of the freeze drying process (see chapter 12.J.3 Validation of the freeze drying process).
Before entering the system, the steam is filtered by particle filters with a pore size of 13 mm and 0.2 mm, warm water (for thawing) is filtered using a pore size of 8 mm and 0.2 mm. The system is ventilated via a drying agent (silica gel) with a vent filter (0.2 mm pore size) in class B. This filter is subsequently subjected to an integrity test.
Once the qualification is successfully completed, the qualification report is compiled and approved (see chapter 6.C.3 Qualification report).
12.J.3 Validation of the freeze drying process
The validation protocol describes the structure, monitoring, and documentation of the validation activities (see chapter 7.H Validation protocol and report). Figure 12.J-9 shows an extract from a validation protocol for freeze drying. The following is an example for the description of devices and process and for the execution of validation.
12.J.3.1 Description of equipment and process
The freeze dryer supplied by XYZ is installed in building 000 in cleanliness grade XY. In freeze dryers for aseptically processed solutions, the loading openings of the drying chamber are in cleanliness grade B. The rest of the machine is located in a lower grade, which facilitates technical monitoring.
| Extract from a validation protocol |
|
|---|---|
Responsibilities |
|
Person responsible for validation |
Head of Production, Head of Quality Control |
Validation manager |
Project manager |
Validation team |
Staff from the unit (users) |
Responsible for implementation |
Staff of the unit (users) |
|
|
The freeze drying process is carried out in accordance with a company SOP. The general structure of this type of SOP is described in figure 12.F-12.
The freeze drying process is executed using pressure and temperature regulation and is recorded using multichannel recorders. More detailed technical information is available in the qualification documentation (electrotechnical descriptions of the control loops).
The three validation runs for each loading configuration and drug-product-specific requirements (eutectic temperature, permitted residual moisture, appearance of the lyophilisate) follow the defined sequence of operation as described in figure 12.J-10.
| Sequence of operation |
|
|---|---|
Program selection |
Automatic/manual start |
Freezing |
Cool trays (approx. -40 °C) Load trays Close system |
Main drying |
Condenser cooling (approx. -50 °C) Switch on vacuum pump Open the intermediate valve Pressure range regulation Tray temperature regulation Tray temperature>0 °C |
Final drying |
Pressure range regulation Tray temperature regulation up to the set end temperature |
Pressure increase measurement |
Pressure increase in the chamber after shutting off the ice condenser using the intermediate valve. Target value: close to 0 |
Ventilation |
Final ventilation using drying agent via sterilised membrane filter with a pore size of 0.2 mm. |
12.J.3.2 Executing the validation
The scope of the validation includes checking all loading configurations that are possible in routine operation according to the specified construction of the machine. In particular, the intensive contact with the cooling surfaces (trays) is tested at all points in order to ensure even freezing and hence formation of uniform "ice bodies" in each container.
The temperature differentials of the cooling and heating medium at the entrance to and exit from the trays should be minimal. This is achieved by uniform loading of the trays and sufficient flow of medium.
The low temperature to be reached by the trays must be adapted according to the requirements of the drug product. The electrical resistance in the drug product should be recorded as a measure of crystallisation or recrystallisation processes, and incorporated in the operating program as a control variable.
- Switch on the register and control devices
- Set the working pressure, for example to 0.2 Torr (2.7 x 10-1 mbar) for a product temperature of approx. - 25 °C. This corresponds to a steam saturation pressure of 0.5 Torr (7 x 10-1 mbar).
- Start the circulation of the heat transfer medium and temperature controller (target values are taken from the program control for heating and cooling).
- Condenser cooling (program control)
- Vacuum pump/s (program control)
- Vacuum control via valve regulation at switching points specified in the program. Pump valve closes = Pressure increases until it reaches the upper switching point -> Pump valve opens = Pressure decreases to the lower switching point = Pump valve closes, etc.
- View the objects visible in the observation window to check the appearance of the lyophilisate.
- Maintain time schedules and temperature increase steps (deviations from temperature and pressure courses due to technical faults are detected by the program control and product protection is initiated by tray cooling that starts automatically).
- Pressure increase is measured once the end temperature is reached.
- System is ventilated.
The documentation includes the following documents:
- Determination of water content of the freeze-dried drug product
- Time-temperature-pressure protocol of the sensors installed in the freeze dryer.
- Evaluation of the time - pressure - temperature course data in accordance with company SOP, and checking of the recorder charts.
To confirm the aseptic working conditions, after the containers are filled with nutrition agar, these are placed in the freeze dryer. All processes such as transport to the freeze dryer, placement in the freeze-drying chamber, withdrawal following a short standing time, are executed, except for freezing. The system is evacuated at room temperature to approx. 100 mbar (to simulate gas transport). The system is then ventilated via a 0.2 mm membrane filter, the removed objects are sealed, and incubated as normal.
The system is cleaned by wiping the surfaces of the freeze-drying chamber with disinfectant and subsequent sterilisation of the chamber and the condenser in accordance with a company SOP.
The results of the tests must be compiled in a validation report. After successful execution and positive assessment of all results, the freeze dryer can be released.
Summary: In freeze drying, products that are unstable in water are frozen and water is extracted from them by means of sublimation in a vacuum, until a specified product-specific residual moisture is reached. Because the products are usually sterile products that cannot be subjected to heat sterilisation, this procedure must be carried out under aseptic conditions. It is important to ensure that a uniform cake is formed that can easily be re-dissolved. The freeze-drying process is controlled and monitored by measuring the temperature, conductivity, time and pressure using calibrated measuring devices. In validation, the individual phases, from freezing, drying and pressure increase, through to ventilation, are inspected. The aseptic conditions are confirmed by a simulation of the process (without freezing). |