Prevention of cross-contamination
Here you will find answers to the following questions:
|
Cross-contamination is described as "[...] the contamination of a starting material or a product by another material or product [...]" (5.18 EU GMP Guideline).
"[...] This risk of accidental cross-contamination arises from the uncontrolled release of [...] materials and products in process, from residues on equipment, and from operators' clothing. [...]" (5.18 EU GMP Guideline). One of the central aims of the GMP specifications is to minimise these dangers. A range of aspects must be considered in order to achieve this objective.
11.J.1 Rooms and facilities
To prevent contamination from dust, it is sensible to reduce the development or release of dust to a minimum. To this end, closed systems should mainly be used. This entails e.g. the encapsulation of machines in which open processes are in progress. Each additional item of pipework etc. increases the amount of cleaning required - a discrepancy which must be evaluated based on a risk analysis. Aspiration is an additional important measure for preventing the transfer of dust although it must be ensured that exhaust systems themselves do not become sources of contamination. Care should generally be taken to prevent fluctuations in the existing negative pressure that would cause a reverse discharge of dust from exhaust systems. Flexible hose systems that are often used must be kept sufficiently clean or be replaced. Monitoring should include checks on the effectiveness of measures implemented.
The requirement for sufficient space (3.8 EU GMP Guideline) can certainly be explained in light of the need to prevent the problems mentioned above. Rooms that are narrow and/or unclearly laid out do not lend themselves well to effective cleaning or efficient separation of products or containers.
Packaging processes are particular sources of danger as they normally take place in central locations. In these areas, different products are simultaneously packaged in different ways near one another. This places demands on rooms, facilities and the process organisation. The line clearance fulfils an essential prerequisite for the prevention of contamination and cross-mixing (see chapter 13.B.2 Line clearance.
The conversion of materials in a conversion zone normally involves transfer on in-house aluminium or plastic pallets. These should be cleaned after use or at regular intervals. The condition of the pallets should be checked during cleaning. Pallets with damaged surfaces (e.g. cracks) must be rejected (see figure 11.J-1).
Waste must be disposed of as quickly as possible. Production waste must be collected outside the production area. This must be ensured as part of the organisational process.
11.J.2 Cleaning
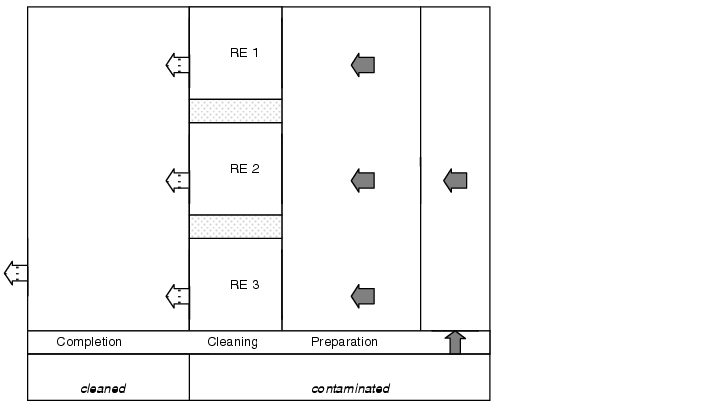
Equipment (such as product containers) that is not cleaned directly on-site should be stored provisionally in such a way that the release of dust is prevented. This may be achieved by designating a room especially for the storage of contaminated equipment that also acts as an anteroom for a cleaning unit. Ideally, the material routes of cleaned and contaminated equipment should be kept completely separate. A linear material flow should therefore be ensured (see figure 11.J-2).
Cleaned equipment and containers must be appropriately stored and protected from the accumulation of dust. For small parts, this may be achieved, for example, by packaging them in polyethylene (PE) bags. Whenever possible, cupboards should be set up outside the rooms in which production takes place. The use of open shelving for storage must be avoided as there is a risk of dust accumulation.
The importance of product-specific cleaning in accordance with optimised cleaning procedures, which have been checked by means of cleaning validation, should be taken into consideration (see chapter 8.B.2 Compilation of cleaning instructions). It is recommended that visual inspections of equipment, machines, containers and rooms be documented prior to use, e.g. on the cleaning label and in the batch record. Consistent application normally leads to an increased awareness of these requirements amongst staff. More often than not, it is smaller, more insignificant, items that can cause contamination, such as adapter sockets for tubes or pipe connections, scoops, small measuring containers, etc. (see chapter 11.C.2 Cleaning and chapter 11.H Identification).
11.J.3 Labelling
The labelling of equipment and containers is extremely important, particularly with respect to the consistent declaration of the cleaning status (see chapter 11.H Identification). They must always be labelled before being used in the production process. Start-ups from equipment that is not classified as acceptable products for safety reasons must be declared to prevent incorrect assignment to acceptable goods.
11.J.4 Personnel
The suitability of working clothing is also important in this regard. The re-release of particles and dust must be regarded as critical. Outside pockets are a potential source of danger in every cleanliness grade (see chapter 11.B.1 Clothing). The transfer of dusts due to personnel movement should be minimised by cleaning rooms and corridors regularly. Dust trap mats at the transition areas between rooms and in locks help minimise the spread of dust from such rooms and must be cleaned regularly.
Training should be carried out on a regular basis to make personnel aware of the consequences of cross-contamination, misidentification and cross-mixing. Regular analysis via discussions on this subject helps pinpoint company-internal weaknesses and allows suitable remedial measures to be taken. (See chapter 2.C Training.)
11.J.5 Reviewing the measures
"Measures to prevent cross-contamination and their effectiveness should be checked periodically according to set procedures." (5.20 EU GMP Guideline). Checks of this kind may be carried out as part of the self-inspection (see chapter 18.E Self-inspection). The check points are then established so that an evaluation may be carried out for the period concerned.
11.J.6 Manufacture of critical products
Specific products should be processed in "dedicated and self contained facilities" (3.6 EU GMP Guideline). These include "highly sensitising materials (e.g. penicillins) or biological preparations (e.g. from live micro-organisms)". The contamination risk associated with such products implies the need for a dedicated, separate manufacturing site. If this manufacturing site is located at a company's premises where conventional production is also carried out with similar logistics, it must be ensured that equipment cannot be mixed up. Separate ventilation systems should exist. At the concept stage, care should be taken to ensure that the air discharge and air intake of separate units are not located near one another (see chapter 3.H Heating Ventilation Air Conditioning (HVAC)). The risk to other products must be regarded as very high. Special attention should be paid even to communication media, in some cases hollow-tube post systems are therefore unacceptable. As they are kept near the product, only copies of the batch records should leave the area (e.g. via fax) to prevent the dissemination of dust. The use of an electronic documentation system is advantageous. (See chapter 15.C Batch documentation)
"[...] certain additional products, such as certain antibiotics, certain hormones, certain cytotoxics, certain highly active drugs and non-medicinal products should not be conducted in the same facilities." (3.6 EU GMP Guideline). In such instances however it is possible to employ conventional production options during the course of manufacturing campaigns. Special precautions should be taken. These include the special labelling of manufacturing equipment or rooms in addition to facility-related precautions.
The risk posed by critical processes can be reduced by segregating them either physically or temporally. A cabin enclosure is an effective means of achieving physical segregation. In this case, the effectiveness of the cabin enclosure measures must be checked during qualification. The area of air-conditioning technology (air exchange rate, pressure differential, proportion of fresh air to recirculating air (see chapter 3.H Heating Ventilation Air Conditioning (HVAC))) is especially relevant. In so-called clean corridors, the corridor in front of the production cabin has a positive pressure in relation to it which minimises the spread of dust from the cabin. The quality of the corridor (no contaminated equipment or other potential dust sources present) and efficient performance of the door-closing mechanism (short opening times) are basic requirements (see chapter 3.E.2 Doors and windows). The risk of dust being carried out via clothing can be countered by wearing special outer clothing and by cleaning shoes. The use of dedicated equipment should be anticipated in many cases particularly if the pharmaceutical ingredient is highly potent and the cleaning is classified as critical (e.g. textile filter bags from fluid bed equipment, tubes that come into contact with products).
Special protective clothing for personnel (in the interests of health and safety at work) should be left in these areas, i.e. a protective suit worn over normal clothes must be removed in the designated area and not outside it. The contaminated suits are wrapped up, e.g. in bags, and then removed. Training has a significant role to play in this regard. Emphasis should be placed on the cleaning of normal clothes prior to leaving the area (e.g. shoe soles: sole cleaning devices and dust trap mats may prove to be useful). It must be clear to staff that simple oversights (such as the removal of breathing masks prior to cleaning) may endanger other products being manufactured. Steps must be taken to ensure that protective equipment does not become contaminated, for example when large quantities of dust are produced. This can be achieved by compartments and cupboards.
"[...] The manufacture of technical poisons, such as pesticides and herbicides, should not be allowed in premises [...]" (3.6 EU GMP Guideline). As a consequence, pesticides, herbicides etc. must not be manufactured in premises that are used to manufacture medicinal products. General checks should be carried out to verify whether the manufacture of a given product influences the manufacturing process and overall quality of another product manufactured nearby. This proximity could be of a physical as well as temporal nature. These rules also apply for the equipment used as well as the rooms: "Normally, the production of non-medicinal products should be avoided in areas and with the equipment destined for the production of medicinal products. " (5.17 EU GMP Guideline). The manufacturing of food or cosmetics is undoubtedly less critical. In terms of organisation however, this should be separated from pharmaceutical production.
Summary Cross-contamination can be prevented by a number of different measures that must be coordinated with one another. One of the main rules to be observed is the temporal and/or physical segregation of different products during their manufacture and the cleaning of rooms and facilities. Critical products such as antibiotics, hormones and zytostatics must be manufactured in separate rooms. |