Weigh-in
Here you will find answers to the following questions:
|
The weigh-in process has a significant, quality-determining importance for the manufacture of pharmaceutical products. The aspects described in figure 11.G-1 must be guaranteed.
| Requirements of weigh-in |
|---|
|
11.G.1 Legal principles
Requirements for rooms
"Weighing of starting materials usually should be carried out in a separate weighing room designed for that use." (3.13 EU GMP Guideline). This explicit requirement for a weigh-in area reflects the importance of the process. In addition to the requirements regarding layout, surfaces, etc., the rooms should also be separate from the other rooms in the production area. During the planning phase, the location of the weigh-in process should be established depending on the defined material and personnel flows. Permanent weigh-in in multi-functional rooms is thus not recommended. This is understandable, as the weigh-in system must be very precisely defined with balances and processes, in order to prevent cross-contamination, mix-ups or confusion.
The layout of weigh-in rooms depends on the material flow, the inclusion in zonal concepts and lock orientations.
To prevent dust production, adequate aspiration must be ensured, at least at the open container and at the balance.
Requirements of balances
Balances and measuring devices must have the appropriate measuring range and required precision (3.40 EU GMP Guideline). They must be calibrated regularly and this must be documented (3.41 EU GMP Guideline). Due to the importance of the initial weight for the subsequent processes and for the quality of the final product, the checks should be carried out frequently, i.e. in line with the utilisation of the weigh-in area. Usually, daily performance testing should be carried out, in addition to the calibration to be carried out in longer intervals. For balance faults discovered retrospectively in the course of the day, the number of critical initial weights can be reduced until the time of the performance test (example: monthly: calibration, daily: performance test with 3 different weights within the calibration range). Calibrations and performance tests are documented in the log book. (See chapter 14.E Calibration in the lab.)
The permissible tolerance must be specified for the respective weighing range, taking into account the measurement inaccuracies, i.e. the tolerated deviation from the target value.
The equipment and utensils used when handling the raw materials must meet the requirements made of surfaces in pharmaceutical production. These must be taken into account when selecting product contact parts, such as scoops (welded seams between handle and pan, rivets, etc., which make cleaning difficult), dosage systems (dosing augers), (pneumatic) loading systems and couplings (see figure 11.G-3).
According to the amendment of the Weights and Measures Act in 1992, balances used in production for weigh-in purposes no longer have to be gauged if regular calibrations ensure that measuring accuracy is guaranteed. However, it can be advisable to gauge the balances in the weigh-in area, in order to enforce complaints against the vendor regarding under filling of API containers.
11.G.2 Weigh-in principles
A distinction can be made between product-specific (i.e. order-specific) weigh-in of different raw materials or raw material-specific weigh-in for different orders. This selection affects the size of room required. Only the raw materials belonging to the weighing order should be found in the weigh-in room itself.
| Weigh-in principles |
|
|---|---|
|
Additive, individual |
|
Individual |
|
|
Product-based weigh-in
Additive and individual (separate) weighings are possible for the product-based procedure, i.e. the starting materials are either successively weighed one on top of the other or individually in containers. To improve productivity, the entire starting material should be quickly available. The procedures make different requirements of the spatial circumstances.
- Additive weigh-in
The advantages of additive weigh-in (space-saving, fewer containers required, less withdrawal loss, as only one individual container) are contrasted with the disadvantage of a possible weigh-in error. Overdoses usually lead to destruction of the container content. If the overdose is only slight, an increase in the other materials can be considered for expensive starting materials. However, this must always be considered in the context of the validated batch sizes. As this is a deviation from the manufacturing instructions, this change must be approved by the head of production. Furthermore, the joint weigh-in of APIs and excipients can cause problems in relation to the definition of the date of manufacture or, as a result, the expiration date and thus the running time of the final product. The appendix to the Note for Guidance on the Manufacture of the Finished Dosage Form, CPMP/QWP/486/95, Start of Shelf-life of the Finished Dosage Form gives the manufacturing date as the time of combination of the API and excipients. Weigh-in at the end of the month can therefore lead to a reduction in the running time of the product by one month compared with an individual weigh-in, if the processing takes place in the next calendar month. A possible remedy is the separate weigh-in of APIs for the additively weighed excipients. - Material-based dimensioning
The actual weigh-in room can be dimensioned so that only the weigh-in process of one material can be executed in it. This reduces the cleaning effort required. Provision area for the starting materials should be directly in advance of this room, in order to minimise the material flows. If further orders are held in supply in this provision area, corresponding assurance of the correct assignment must be guaranteed. As the cleanliness grade increases, the weigh-in room design for smaller rooms (cabins through to LF benches) is simpler in terms of environmental conditions, air flow patterns and monitoring, compared with large rooms. The use of this room layout is primarily of interest for the processing of a few different starting materials. - Order-based dimensioning
Product-specific weigh-in of a large range of starting materials can also be carried out in large rooms (depending on the cleanliness grade). In this case, provision for the weigh-in process in the weigh-in room is order-specific. This requires organisation within the room and of the processes. It is possible to assign areas within the room, e.g. storage of the starting material containers on one side of the room; then the balances with different weighing ranges on the other side of the room, separated by a corridor. The hazard of containers of other raw materials becoming contaminated can, in addition to the spatial orientation, be minimised by specific air supply or air extraction flows (e.g. LF principle, directional aspiration on balances and over open containers). In this way, the effort for cleaning can also be reduced.
Raw material-specific weigh-in
For raw material-specific weigh-in, the dimensioning of the weigh-in room is mainly determined by the volume throughput, i.e. the size of the delivery containers (Big Bags) and the material flow (horizontal or vertical).
Central/decentralised weigh-in systems
Central and decentralised weigh-in systems are possible. The optimal solution for the plant is to be viewed according to the spatial circumstances, material flow, volume throughput and variability of the starting materials. Often, central weigh-in areas are used with supplementary island solutions, i.e. further weigh-in areas as satellite systems.
11.G.3 Weigh-in procedure
A distinction must be made between manual and automatic weigh-in. The use of such a procedure depends on the company-specific requirements. Often, it is advisable to supplement automatic systems with smaller volume, manual weigh-in.
| Properties of manual and automatic weigh-in procedures |
|
|---|---|
Manual weigh-in |
|
Automatic weigh-in |
|
Manual weigh-in
Manual weigh-in is mainly used if there is a high diversity in the materials to be weighed in, as well as a low to average volume throughput. It offers the advantage of higher flexibility and a lower effort for cleaning.
Automatic weigh-in
In principle, automatic systems can be vertically or horizontally organised. Various aspects are important for the selection and operation of an automatic weigh-in system:
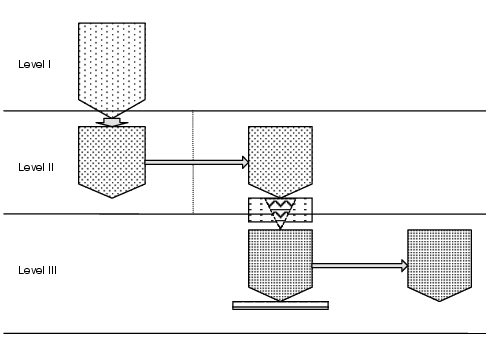
In automatic weigh-in systems, the transport, filling, dosing, further transport of starting materials and containers depends on the degree of mechanisation. There are different dosing principles (mobile balance, weigh-in funnel, etc. as well as direct weigh-in or with preliminary ensiling or sieving steps). Manual operations are also frequently found in combination with automatic weigh-in processes. Smaller sub-quantities are then dosed in via the control system. The requirements of the facility design are determined by the type and volume as well as the variety of raw materials. Figure 11.G-6 shows a vertical material weigh-in.
In level I, the starting materials are delivered, e.g. in Big Bags, and converted to level II. In level II, additive dosing is carried out via mobile containers (level II-III) into e.g. containers (level III) that are then incorporated into the production process. The dosing procedure is checked or controlled via a (mobile) balance in level III. Transitions between cleanliness grades must be taken into account.
| Aspects of automatic weigh-in systems |
|---|
|
To reduce cross-contamination, it is extremely important to prevent dust production. Dust-tight docking systems on containers, aspiration facilities (ring aspiration) help minimise the risk. Particular attention is to be paid to aspirations and air handling on the dosage units. It must be ensured that backwards contamination of the dosage units through dust production from the filling process is minimised.
11.G.4 Weighing process sequence
All processes from delivery to return must be fixed in written procedures in order to ensure that they can be controlled.
The weighing process may be preceded by various processing steps, e.g. sieving steps (as protection or classification sieving) or ensiling steps.
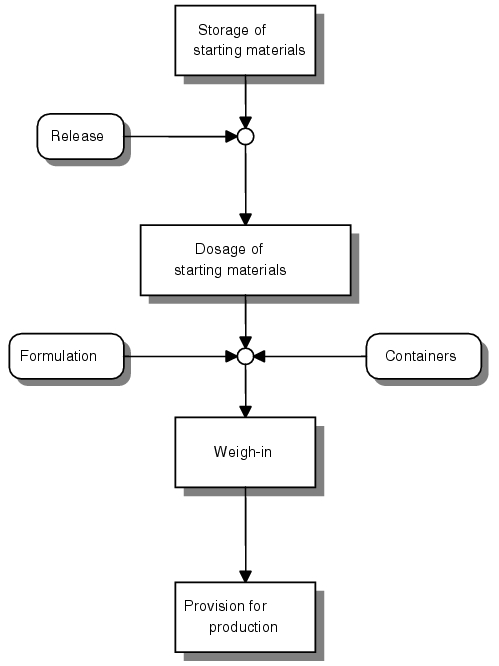
The main sequence of the weigh-in process is illustrated in figure 11.G-8. It is important that the dosage factors be compiled when the starting material is released, so that fomulation-compliant and specifications-compliant quality can be produced.
Provision for weigh-in
This takes place after conversion of the raw material (either from the storage area or areas with a lower cleanliness grade) to a provision area. Here, the containers are checked for identity, cleanliness and integrity. For product-based weighing, a complete order should be delivered from the storage area if there are several components. This means that pallets or other delivery forms should be secured individually after picking, e.g. through pallet cages. This will prevent confusion if containers fall, with subsequent incorrect assignment and unauthorised access.
Weigh-in
Only suitable and authorised persons may be involved in the weigh-in process. Reliability is of the utmost importance in this critical area. Competence can be expressed in the assignment of user rights for the weigh-in systems used.
In principle, a distinction can be made between weigh-in with or without EDP support. The following versions are based on manual weigh-in, to give an overview. The principles can be applied to automatic processes.
Weigh-in without any EDP support should now only be used in exceptional cases. The second set of eyes principle applies here, i.e. the activity of the person carrying out the weighing is checked by a 2nd person. For pharmaceutical practice, this procedure bears a high risk potential through transfer or reading errors. When composing the weigh-in regulations, this must be accounted for through a high level of clarity (font sizes, line and column spacing, etc.). Containers must also be identified via a visual control.
EDP supported weigh-in systems reduce the risk in the weigh-in process. The system cannot be used unless it is qualified or validated (see chapter 9 Computer Validation). These weigh-in systems can be used in various controlling levels. This can mean simple administration of weigh-in orders through to complete recording of operating data.
| Aspects of EDP support in a weigh-in system |
|---|
|
- User identification
Various techniques, e.g. chip cards, barcodes on working clothing, alphanumerical inputs, can be used to guarantee the authority of the person performing the weigh-in and to acknowledge activity on superordinate systems. Depending on the EDP standard, simple identification can be sufficient, or an electronic signature might be required. - Container identification
The identification of the container via barcodes, for example, is an additional safety precaution. The barcodes are usually applied upon receipt of the goods as part of goods acceptance. A status request can be executed by coupling the data with the data from the warehouse management system, for example. Thus, the batch or container release can be checked at this stage, directly before weigh-in. This is also possible for the expiry date. Scanning of a container that has not been released, or for which the expiration date has been exceeded, can accordingly lead to cancellation of the weigh-in process. The differentiation of individual containers is a good way to trace back the properties of an API batch. - Assignment of containers
Specific containers can be assigned to the weigh-in personnel for weigh-in, e.g. as part of a product-specific application (dedicated equipment). When recording the operating data, it is advantageous to keep a product history, i.e. traceability of the uses of containers. The requirement to use only suitable and clean containers can thus be easily and effectively implemented, as the cleanliness status is available in the system and thus only suitable vessels can be used. - Balance assignment
It is possible to stipulate the balance used for certain weigh-ins (e.g. balance type or balance number). This can be important for certain weigh-ins, e.g. by stipulating ex-protected rooms, rooms of specific cleanliness grades, weigh-in of raw materials with extreme bulk densities (e.g. Aerosil). - Checking the weigh-in process
The permissible deviations from the target value defined as part of the tolerance definition are directly converted and controlled. Larger deviations are not accepted. Starting materials that are calculated with dosage factors (e.g. to balance out content fluctuations, loss on drying, etc.), can either be converted to the necessary required value in the weigh-in system or the requirement from the superordinate system can be applied. Particular attention must be paid to these points during qualification and validation.
Different display procedures are used:
- subtractive, i.e. the display of the current difference from the required value;
- additive, i.e. the total of the partial quantities already weighed in.
The display of tolerance bars, possibly highlighted in colour (red/green) is a valuable orientation aid for the balance tolerance.
A quantity outside the upper tolerance level, which is taken from the original container, must not be returned to this container.
Return
Leftover containers are usually sent to the warehouse via provision. The container must be appropriately and sufficiently sealed, i.e. in terms of tightness and with no influence on the quality through adhesives that might come into contact with the product. Furthermore, it must be ensured that no product dust gets onto the exterior of the container. This is due to safety at work, but also to prevent dispersion effects and cross-contamination.
Provision for production
The EU GMP Guideline permits short-term interim storage in the production area. The weighed starting materials should be processed as quickly as possible. This is comprehensible, for stability and running time reasons. Care must be taken that the container cannot become dusty, as this could endanger the product quality during disposition (e.g. when emptying). The requirements of the storage method are the same as those for provision for weigh-in. Long standing times should, if necessary, only be allowed in the storage area (with sufficient enveloping).
Cleaning
Balances and rooms are cleaned after each weigh-in in order to avoid carryover to the next order. The bases of the balance bridges or balance cavities must also be cleaned regularly as there is an increased risk of microbial contamination here. Cleaning is documented in cleaning records. Microbiological loads and particle loads must be monitored regularly. Starting materials may have a high microbial count due to their origin and based on the microbiological limits permitted by the pharmacopoeia (see chapter 11.E Environmental monitoring). Therefore, product-specific disinfection methods may be necessary for the entire room, let alone for the weighing area. All cleaning and disinfection measures are to be codified in the room cleaning records or log books (see chapter 11.C.2 Cleaning).
Documentation
Print no. |
Place of work |
Weigh-in: FSB |
Container |
17-51-0 |
20-2-00 |
17-51-3 |
17-51-5 |
|
Balance |
12-23-2 |
12-23-1 |
12-23-2 |
12-23-2 |
||||
GMP Pharma |
Work step/ |
Initial weight/ |
Date/ |
17.03.99/ |
17.03.99/ |
17.03.99/ |
17.03.99/ |
|
Batch size 460.00 |
User |
müller |
müller |
müller |
schmidt |
|||
Unit |
kg |
kg |
kg |
kg |
||||
Logo |
Batch |
4563A1 |
Tare |
10.45 |
1.040 |
10.34 |
10.41 |
|
Actual |
100.10 |
9.99 |
75.00 |
50.07 |
||||
Material no. |
123456 |
Batch no. 1 |
Converted |
100.00 |
10.00 |
75.00 |
50.00 |
|
Dosage factor |
1.00 |
1.00 |
1.00 |
0.90 |
||||
Material |
Granulate test |
Batch quantity 230,00 |
target |
100.00 |
10.00 |
75.00 |
45.00 |
|
Batch |
001A5 |
154b1 |
147c2 |
178a8 |
||||
Order number |
4563139 |
Material |
12345 |
23452 |
13541 |
16444 |
||
Material |
Lactose |
Ethanol |
Cellulose |
Potato |
||||
Item No. |
1 |
2 |
3 |
4 |
Weigh-in is carried out in accordance with the manufacturing instructions. Records of the weigh-in process are required, which stipulate the correct manner of execution. This means that information on materials, batch IDs, required quantities, actual quantities, possible dosage factors, weigh-in date (possibly time) and the name of the person performing the weigh-in must be available. In the case of electronic batch recording (see chapter 15.C.3 Electronic batch recording) the paper form can be omitted.
Containers must be labelled. The content must be unambigously identifiable.
When using EDP supported systems, the printout of records and labels is generated by the system. Printers within the weigh-in room must be enclosed in a housing in order to prevent any mutual influences (dust production/degassing).
Labels should be applied to the containers immediately in order to avoid confusions after completion of one weigh-in process and before another.
Summary The weigh-in process is a sensitive point in the process chain. The hazard of cross-contamination and confusion must be minimised through structural and organisational requirements. A distinction is made between additive (all raw materials for one order in one container) and individual (all raw materials individually) weigh-in. Moreover, depending on the number of raw materials used in the company, a distinction must be made between order-based or raw material-based weighing. EDP supported, validated systems offer a high level of security: Only the raw materials clearly specified in the manufacturing instructions and only released containers can be weighed. |