Hazard Analysis of Critical Control Points (HACCP)
|
This section addresses the following questions:
|
The HACCP was developed in the early 1960s as part of the NASA program in order to manufacture foodstuffs for use in the space program with a 100% guarantee against contamination by bacteria, pathogenic viruses, poisons or chemical and physical hazards. The HACCP replaced end-product testing and at the same time provided a guarantee for the safety of foodstuffs. Since then, the HACCP has become recognised as an efficient tool for quality assurance in the foodstuffs manufacturing industry.
The HACCP concentrates on the aspect of hygiene, which is of vital importance for any operation involved in food processing. Legislators have also recognised the significance of hygiene in this area. The introduction of a legal obligation for food processing companies to perform an HACCP represented an important milestone for this method (EC Directive 93/43 on the hygiene of foodstuffs). The requirements in § 4 of the hygiene regulation mean that this form of risk analysis has become the standard in this industry.
The HACCP concept has developed beyond the food technology industry, where it considers direct hygiene aspects, to become an established method for the execution of risk analysis in the pharmaceutical environment.
The acronym HACCP covers the activities of a risk analysis: It consists of
- an assessment of potential hazards (hazard analysis) and
- the determination of critical points in a process which enable regulatory or controlling intervention (control points), in order to prevent the hazard or reduce it to an acceptable level.
This method of hazard analysis with the monitoring of critical control points (CCP - Critical Control Point) results in a preventive monitoring system and is an effective concept for quality assurance in terms of both hygienic and technical parameters that may influence product quality. Furthermore, the risk analysis is also extended to include the inspection of documents such as manufacturing and test procedures, specifications, operating instructions (SOPs), method validations, application files for marketing authorisation and laboratory reports. The analysis of the required documents and the suggested manufacturing process help in the preparation of a product-specific validation protocol.
Just as for the FMEA and FTA, the HACCP should also be performed in a multidisciplinary team. It is recommended to include members from the areas of engineering, analytics, biology, production, hygiene, validation and marketing authorisation. The task of the team should be to provide the necessary resources for a complete analysis of the available and potential risks, and subsequently for the implementation of measures.
The formalised HACCP concept is based on the inspection and evaluation of processes/processing steps based on seven different levels. Each (sub)step in the process is evaluated against each of the seven aspects.
The seven levels are as follows:
1. Execution of hazard analysis
2. Identification of critical control points (CCP)
3. Determination of critical limits
4. Monitoring of the critical control points
5. Definition of corrective actions for values above the threshold (OOS results).
6. Documentation of the processes (for steps 4 and 5)
7. Verification of the operability of the system
1 Prerequisite and result
In order to apply the HACCP concept, a preliminary description of the process is first required. This includes at least the product descriptions including composition, the processing steps from manufacturing documents, the materials used, the production machinery and premises and the environmental conditions (hygienic areas). We recommend that you compile the process description in graphical (e.g. flow chart) or tabular form. You can then begin the actual risk analysis according to the HACCP (steps 2-7).
The HACCP method delivers the following results:
- The HACCP enables the assessment of potential hazards (hazard analysis).
- Critical control points and the acceptance criteria are defined.
- Suitable corrective action can be planned and applied in the case of deviations.
2 Execution
The seven steps of the HACCP method are described in more detail in the following:
2.1 Step 1: Analysis and identification of potential risks
(hazard analysis)
In this case, each step in the whole manufacturing process from the beginning (starting materials and raw materials, primary packaging materials, etc.) to the finished product is inspected for potential risks. This type of risk analysis (e.g. using an FTA or fishbone diagram) is specific to different products and procedures and should include (at least) the following aspects (figure 1):
|
Building/space conditions (environmental conditions, hygiene aspects) |
|
|
Personnel |
|
|
Technical system aspects (quality/contamination risks) |
|
|
Manufacturing and development |
|
|
Material |
|
The relationship (traceability) between the process schema (first level) and the HACCP table (figure 1) is guaranteed by a unique number (e.g. see figure 3 and figure 4 in chapter 4.2 Documentation).
Two subsequent steps are required to enable unique assignment of the substeps and their risks:
- The lower-level processing steps or the equipment/facility functions are listed in a second column (second level).
- The subprocesses or the affected equipment/facility components are listed in the third and final level.
The risk analysis that is now carried out can be performed as a Yes/No decision or separately, e.g. using an FMEA.
2.2 Step 2: Determination of the critical control points
(Critical Control Points, CCP)
Critical control points are the local conditions, practices, activities or procedures that require regulatory intervention in order to reduce or prevent a recognised risk. "Control" here is used in the sense of "keeping under control" rather than "testing". A processing step or a raw material, etc. must be evaluated to assess whether the potential risks can be managed with the use of control mechanisms.
It can be very useful to compile flow charts; this makes processes and interrelations more transparent and thus shows a certain correlation to the FMEA and the FTA.
The relationship and the mutual dependence of process (manufacturing process) and environmental conditions (environment influences) on the desired product quality and hygiene situation must be analysed in order to enable subsequent breakdown of this general subdivision into directly (and indirectly) involved process and environmental factors.
A risk evaluation must be executed for each individual process and environmental factor. The aim of this evaluation is to identify risks, describe the problem, (e.g. in order to communicate or provide training in it), evaluate the problem, eliminate the problem, or, if this is not possible, initiate suitable control mechanisms (e.g. through descriptive instructions, SOPs).
The formal route of the HACCP concept enables each individual processing step or component to be highlighted in terms of product-specific or general (e.g. facility-specific) and, in particular, hygiene risks. A procedure of this nature also means that influencing variables of potential risks can be evaluated, warning or threshold values for control experiments can be defined and suitability criteria can be specified.
Risks - not necessarily only hygiene risks in the most exact sense - must be integrated, evaluated and controlled. Influencing variables receive a relative weighting, which means that the HACCP team takes into account the demands of different departments, as shown in figure 2.
|
Manufacturing staff |
|
|
Person responsible for hygiene |
|
|
Validation team |
|
|
Biologist |
|
|
Analytical scientists |
|
|
Staff from |
|
|
Technicians |
|
The HACCP team must be capable of evaluating the different aspects that need to be taken into account.
If risks cannot be completely eliminated, downstream control strategies must be developed and established. The aim must always be to ensure the safety of the whole process.
2.3 Step 3: Determining the Critical Control Points (CCP)
If the critical parameters can be granted a certain level of tolerance, the corresponding tolerance limits must be clearly defined and, if necessary, justified.
Limits are used as criteria to evaluate whether a process (step) is under control at a particular control point. To enable early and stepwise handling, warning values and alarm plans for exceptional events must be defined.
The effectiveness of this type of defined measures must be tested ("validated"). The limits (threshold values), e.g. for physical, chemical, or (micro)biological variables must be specified (temperature, time, water activity aW/pH value, etc.) and evaluated in terms of significance (e.g. in case the threshold value range is too wide).
2.4 Step 4: In-process control, monitoring of critical control points
Permanent monitoring of the limits set in chapter 1.11.2.3 Step 3: Determining the CCP fixed values and the procedural conditions in terms of controlling processes (control) that are already running (in process), provides good protection against a "creeping" effect of the process and hence against the eventual materialisation of risk situations. For monitoring limits, process control cards with warning and intervention thresholds can be used very effectively.
In-process control includes observation of the set critical limits and should be regarded as a warning process that enables the correction of processes that have run out of control while they are still running (e.g. method analytics, levels running empty below a fill line after long downtimes, pressure and coding colour quality, etc.). The threshold values and their significance also require evaluation.
The statistical processing of the organism and particle status over long periods of time and under production and rest conditions of premises, personnel, mechanical equipment, the production environment and the process water lead to a precise knowledge of the hygiene status of the production factors. Trend observations enable the timely introduction of countermeasures. This means that a preventative, stepwise reaction to changes is possible. Risk prevention and control is preferred to damage limitation.
In-process control is therefore the decisive step and represents the core of the HACCP concept. You need to analyse who can implement or perform corrective actions, when and how competently.
2.5 Step 5: Determining corrective action
The observation of the specified critical limits should be regarded as a form of warning process. Corrective action should be taken during ongoing operations as soon as the in-process control shows that the process is out of control at a critical control point.
If the measured values show a collision with the tolerance limits or with the limits of the control cards, targeted measures should be implemented to ensure that the average process conditions become closer to the required value.
These measures should be evaluated to check whether they are capable of returning the process to an acceptable status within a fixed timeframe It is important that exact instructions exist regarding the corrective action and that these measures are documented.
2.6 Step 6: Documentation of processes
The process or its substeps should be reviewed to ensure whether it can be guaranteed that any findings are collected and stored in a suitable manner and that all required information can be accessed rapidly. All records from the sequence of operation must be collected in order to gain a report synchronised with production.
Particular attention should be paid to any deviations and the measures implemented. The documentation of deviations from the specified process, with their evaluation and consequences (failure frequency) should be evaluated in order to recognise any trends.
2.7 Step 7: Verification of the HACCP system
Following/at the same time as installation of the HACCP system, it is necessary to check (possibly at certain chronological intervals) whether the system is functioning according to its intended purpose.Measures should be evaluated that can be used to test whether the HACCP system (and hence the process) is running smoothly. This type of system control can be enabled, for example, by random sampling of end products or analysis of particular CCPs (e.g. bioburden analysis). The verification also includes inspection of the process environment (chapter 12.G Microbiological monitoring).
3 Advantages and disadvantages
The HACCP method has the following advantages:
- There are many advantages associated with the implementation of the HACCP for risk management.
- It systematically covers all critical control points
- It systematically evaluates all processing steps in terms of the possible risks and specifies measures for minimising risk.
- The HACCP forms the basis for setting up preventive monitoring systems for both hygienic and technical parameters. It therefore guarantees a rapid and precise reaction, e.g. to OOS events.
- It is a comprehensive method that catches all potential failures.
- It provides a valuable basis for a "quantitative" evaluation of risks, e.g. by FMEA.
- It is used to prepare a validation/qualification.
- It utilises synergies through the formation of interdisciplinary teams.
- The process and risk analyses can be used for further optimisation of processes.
- It can also be used for the analysis of non-GMP risks.
The disadvantages are:
- An HACCP is not used for the evaluation or classification of risks.
- Since the main aim of the HACCP is to manage processes, processes must be viewed in their entirety. This requires an exact description of processes with a subsequent risk analysis. If processes are not yet described in sufficient depth, this must be performed prior to the HACCP, which is a time-consuming process.
- Training is required before initial execution.
- Sufficient resources must be made available.
4 Application example
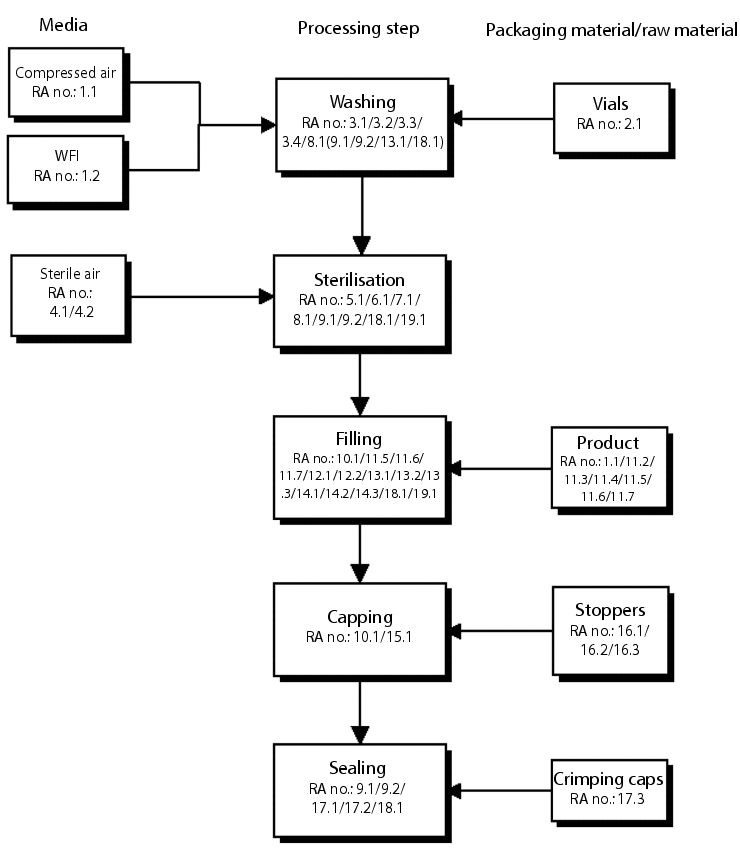
The following is an example of how the HACCP method can be applied. The example contains extracts from an article by M. Jantsch et al., in which the HACCP methodology is applied in order to define the validation measures necessary for the implementation of an aseptic process, the filling of sterile powder in glass vials.
In a multidisciplinary team, the procedure for performing a risk analysis according to the HACCP concept is first explained in detail and then the manufacturing process and its environmental factors are analysed for potential risks that may affect quality. A table is used to identify and evaluate potential risks, define measures for risk reduction and summarise uncontrolled risks in a product-specific validation protocol. Following the risk analysis, the examples of activities derived from this procedure for a product-specific validation protocol, the qualification of renovated premises and room ventilation facilities and the qualification measures for the fill line were described in extracts, with focus on the "Sterilisation" processing step.
4.1 Process description
For more information on the processing of process information, see chapter 10.F.2.1 Preparation of the necessary process information. The process description required prior to the HACCP includes the following aspects:
- Product description including composition
- Packaging, storage and delivery conditions
- Stability
- Preparation instructions
- Flow chart of the manufacturing process
- Documentation of hygienic areas (including neighbouring rooms).
|
Extract from process description for the "Sterilisation" processing step: "For the production of the aseptically filled powder, a compact washing, filling and capping system will be used. The filling and capping machine will be installed in combination with a turntable and a transport system of grade A. Glass vials will be washed with water for injection and then sterilised in a sterilisation tunnel. The vials will be transported to the filling and capping machine via a turntable and conveyor belt. [...]" |
4.2 Documentation
The HACCP concept was applied to all processing steps of the manufacturing procedure, including washing and sterilisation of the primary packaging material, gate control of personnel and materials, disinfection and sterilisation, filling, sealing and flanging, and quality control.
Starting from the manufacturing process schema (figure 3), each processing step was broken down into its individual measures and analysed in terms of the risks to be analysed.
 |
The results were documented in a table (figure 4).
|
Seq. no. |
Equipment/ facility function |
Seq. no. |
Equipment/facility component or function |
Risk in terms of product manufacturing |
Critical |
Measure for risk |
Risk controlled by measure |
Body of rules/ |
||
|---|---|---|---|---|---|---|---|---|---|---|
|
Yes |
No |
Yes |
No |
|||||||
|
Sterilisation |
||||||||||
|
1 |
LF unit |
4.1 |
LF warming zone |
No filter integrity available |
x |
Filter integrity tests |
x |
Equipment specification |
||
|
4.2 |
LF cooling component |
No filter integrity available |
x |
Filter integrity tests |
Equipment specification |
|||||
|
Pressure differential before/after filter too high or too low |
x |
1. Pressure differential display with alarm settings available |
x |
IQ/OQ sterilisation tunnel |
||||||
|
2. Integrated flow controllers that raise the alarm if levels are below the minimum air velocity |
||||||||||
|
5 |
Temperature control |
5.1 |
Radiant heater |
Heater power decreases or is not sufficient |
x |
1. Qualification of the sterile tunnel |
x |
IQ/OQ/PQ sterilisation tunnel |
||
|
2. Registration of temperature at tunnelrecorder |
||||||||||
|
3. Automatic tunnelstop if temperature falls below the minimum |
||||||||||
|
4. Revalidation |
||||||||||
|
5. Function control lamp available for each heater on the tunnel |
||||||||||
|
6. Control of heat distribution |
||||||||||
|
7. Inspection of endotoxin reduction |
||||||||||
|
6 |
Pyrogen removal process |
6.1 |
Sterilisation |
<Microbiological impurity |
x |
High process temperature (> 280 °C, > 3 mins) and inspection of endotoxin reduction in the PQ |
x |
PQ sterilisation tunnel |
||
|
Particle load of vials |
x |
1. Inspection of particle reduction in the purification process |
x |
PQ washing machine |
||||||
|
Glass breakage in tunnel during sterilisation |
x |
1. 100 % visual control |
x |
Qualification |
||||||
|
7 |
Room |
7.1 |
Transport of vials from RK-D to RK-A |
Microbiological contamination risk |
x |
Transport of washed vials through sterilisation tunnel in RK-A and storage in RK-A until sealing |
x |
Culture medium filling |
||
|
8 |
Personnel and facilities |
8.1 |
Procedure |
Contamination risk |
x |
1. Execution of culture medium filling before release of equipment and repetition every six months |
x |
Culture medium filling |
||
|
2. Hygienic procedures by personnel ensured through training |
SOP |
|||||||||
|
3. Cleaning of equipment and rooms by trained personnel |
SOP |
|||||||||
In this example, the relationship between the process schema and the risk analysis (figure 4) is guaranteed by a unique number (1st column "Seq. no."). The "Equipment/function" column lists the function. The subprocesses or the affected equipment/facility components are listed and numbered in the subsequent "Seq." and "Equipment/facility component of function" columns. The existing or potential risk in terms of product manufacturing or equipment/facility function is then determined ("Risk in terms of product manufacturing or function" column). In the subsequent column, the risk is then evaluated to establish whether it leads to impairment of product quality or safety (critical: "Yes") or not critical: ("No"). For all critical risks, "Measures for risk reduction" must be established in the next column. There then follows an evaluation of whether or not a risk is controlled by the established measure. A risk can be controlled by organisational measures (SOPs etc.), validation studies, or through downstream control points (CPs). The final column lists the relevant body of rules (validation studies, SOPs, procedural instructions, etc.).
|
Summary A hazard analysis and monitoring of critical control points (CCP, Critical Control Points) lead to a preventative monitoring system and offer an effective concept for quality assurance. After the process has been analysed for risks (e.g. using FTA or an fishbone diagram), the critical control points are identified and critical threshold values determined. These are monitored by in-process controls, so that the process that is out of control can be corrected while the process is still running. The application of risk analysis according to the HACCP methodology is a suitable procedure for structured recording of potential risks, e.g. in the manufacturing process, introducing the necessary qualification and validation measures, enhancing or creating the required documentation. The risks in an HACCP table should be repeatedly re-evaluated in the form of a life cycle document and the risks classified as critical should be adequately controlled. |

