|
Here you will find answers to the following questions:
|
Optimisation of cleaning procedures
To carry out the time-consuming and expensive cleaning validation successfully, it is essential to optimise the cleaning procedures prior to the actual validation.This applies to both new procedures to be developed and also existing cleaning procedures.
The objective of an optimisation is to develop a cleaning procedure which fulfils the following requirements:
- Effectiveness under worst case conditions
- Cost-effectiveness
- Validatability
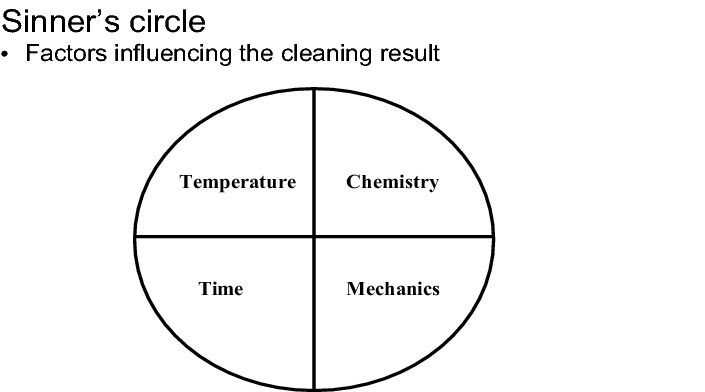
When developing the cleaning procedure it should be noted that the results of the cleaning procedure are determined by four essential influential factors, whose interaction is also described as a Sinner's circle (see figure):
 |
To guarantee effectiveness under worst case conditions, the worst case must be defined in more exact terms. The first thing to be determined is whether all the products made using the equipment can be cleaned according to the same procedure, or whether due to different product properties, several cleaning procedures are required.
Within each group of products, for which an individual cleaning procedure is required (e.g. alkaline-soluble products or acid-soluble products) the product which is the most difficult to clean represents the worst case. If the cleaning procedure is designed in such a way that this product can be cleaned in a reliable manner, the effectiveness of the cleaning procedure is guaranteed for all other products which are easier to clean and are in the same group as well.
With regard to the validation, the establishment of the following general conditions must be integrated in the optimisation phase:
- Establishing a maximum holding time for uncleaned equipment
- Deifinition of maximum campaign length, after which cleaning must be carried out.
Establishing the chemistry, temperature, mechanics and time parameters
The cleansing agent is selected mainly in accordance with the solubility properties of the worst case product. These properties are determined not only by the active pharmaceutical ingredient - possibly contained in small quantities only - but also by all the substances present in the formulation.
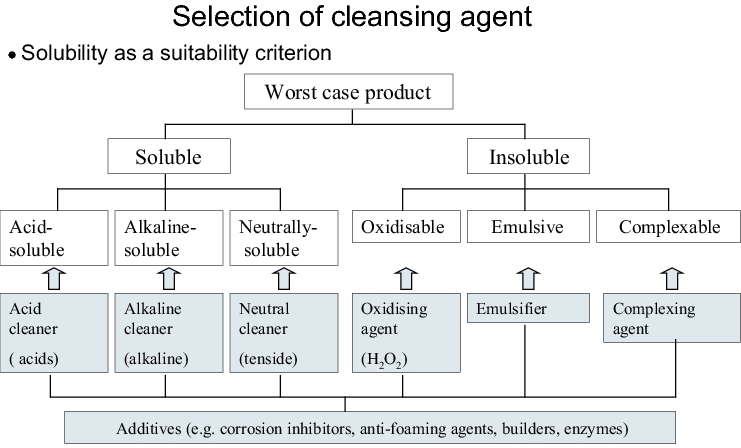 |
In practice, most formulations contain ingredients with different solubility properties which make a combination of different cleansing agents necessary. For this reason, the cleansing agent manufacturers offer both combination preparations (e.g. alkaline cleaners plus tenside) and individual components which can be combined with each other according to the modular design principle. The better the compatibility of the cleansing agent or cleansing agent combination with the contamination, the more effective is its use in practice.
Further criteria when selecting a cleansing agent are environmental and material compatibility, safety at work and price. With regard to the validation, it should also be considered that no superfluous components such as fragrances and dyes are included. It should also be guaranteed that the cleansing agent manufacturer announces the composition of the cleansing agent and gives the pharmaceutical manufacturer information on the critical changes to the formulation (requirement of PIC/S guideline PIC/S PI 006). The cleansing agent and its individual components should meet the requirements of the Food and Goods Law. With respect to this, a document of compliance can be requested from the manufacturer.
Since conventional household cleaners mostly contain fragrances and colouring agents in addition to preservatives, the formulation is often changed several times per year and the scope of documentation needed for the cleaning validation is usually not available from the manufacturer, these cleaners are not recommended for the cleaning validation.
The optimum concentration of cleansing agent can only be established if the other process parameters, i.e. temperature, mechanics and time, are also taken into consideration.
The temperature range within which a cleansing agent will develop its effectiveness to the optimum extent, is normally specified by the manufacturer. In the majority of cases, the effectiveness of a cleaning procedure can be enhanced by a temperature increase. It is therefore recommended to select as high a temperature as possible within the specified range, provided that this can be done whilst taking the technical circumstances and the safety at work into consideration.
The mechanics available vary from equipment to equipment. This generally poses the question of whether the equipment will be cleaned manually or a CIP or WIP system is connected (see figure). With regard to the cost-effectiveness of the cleaning procedure, the mechanics take priority because an efficient mechanical removal of contamination makes it possible to minimise the proportion of the remaining parameters of the sinner's circle.
If the chemistry, the temperature and the mechanics are designed optimally and in harmony with each other, the time factor can be limited to the minimum dimension necessary. It should, however, be noted that only the time the cleansing agent is left to work is of relevance here. The total time taken up by a cleaning procedure is also determined by working steps such as pre-cleaning, disassembly and assembly of equipment parts and the rinsing and drying procedures required for removing the wash water. In practice, these procedures can take up more time than the main cleaning procedure itself and must also be subjected to a critical inspection during the optimisation process.
The following checklist (see figure 8.B-3) summarises shortly all the aspects to be taken into consideration during optimisation.
|
Influential |
Aspects of optimisation |
|---|---|
|
Temperature |
Rule of thumb: As high as possible |
|
Chemistry |
Rule of thumb: As much as necessary, as little as possible |
|
Mechanics |
Manual cleaning:
WIP/CIP cleaning:
|
|
Mechanics |
Rule of thumb: as efficient as possible |
|
Time |
Rule of thumb: as short as possible |
Compilation of cleaning instructions
Once all the details of the cleaning procedure have been firmly established, a written cleaning instruction (SOP) has to be established.
The availability of a written cleaning instruction and its implementation is requested in the CFR, Part 211 in § 67. This section also contains detailed specifications regarding the contents of a cleaning instruction.
The EU GMP Guideline (chapter 3.36) also requests that cleaning is carried out in accordance with detailed procedures, but does not give more precise specifications on the contents or the structure of these cleaning SOPs.
The Cleaning instruction checklist (see figure) can be considered as a suggestion of how to structure the contents of a written procedure, and includes all important details (see figure)
|
Suggested structure for standard cleaning SOP |
|---|
|
I Identification of the equipment |
|
II Critical areas |
|
III Implementation of cleaning The following specifications must be contained in this section:
|
|
IV Documentation |
|
V Checks |
.
Identification of the equipment
This section should contain as accurate a description as possible of the production equipment, by specifying equipment type, manufacturer, location and inventory number, for example. If several equipments of equal type or design are available, these can be summarised in the cleaning SOP and later on in the validation protocol.
Critical areas
The so called critical areas should already be specified in the cleaning SOP. They include individual areas of production equipment which can hardly be accessed during cleaning and/or which are very difficult to see during the final checks. If these areas are already indicated in the cleaning SOP, the staff will already be advised to be especially careful when cleaning and checking the relevant areas prior to cleaning validation. It is advisable to attach a drawing of the equipment to the SOP, on which the critical areas are clearly indicated. If the relevant drawings are not available or if they do not show the critical areas, photographs are a good alternative.
Implementation of cleaning
Cleaning intervals: A complete cleaning of the production equipment is essential in the event of a product change. However, further specifications can also be required, such as:
- cleaning steps which should be implemented on a daily basis during a campaign
- maximum allowable campaign length until implementation of complete cleaning
- maximum allowable holding time for the uncleaned production equipment until complete cleaning
Cleansing agents and concentration of the cleaning solution: A precise description of the cleansing agent must be provided here in addition to the concentration required for application. Instructions for safety at work when handling cleansing agent concentrate or cleaning solution are also required here. To guarantee that the cleaning solution is actually used in the concentration required, the production using a suitable dosage system or dosage appliance should be described. If this produces an undesired redundancy (e.g. because many facilities are cleaned with the same cleansing agent in the same concentration), then the manufacture of cleaning solutions can also be desribed in a separate SOP.
Quantity and temperature of the cleaning solution: The quantity of the cleaning solution is clearly defined in CIP and WIP systems, either through the volume itself (seal housing/circulatory cleaning) or via the sequence time at a constant flow rate (lost cleaning). The temperature of the cleaning solution can also be controlled and checked in a simple, clear manner when using CIP and WIP systems. For manual cleaning, statements concerning the quantity of the cleansing solution and its precise temperature are not always possible. It will be enough to specify, for example, that 10 l hot, 1% cleansing solution shall be provided in a container for cleaning.
Mechanics: For CIP and WIP systems, the spray heads or cleaning nozzles to be used as well as their positioning in the equipment must be specified. For manual cleaning, the devices to be used, such as sponges, brushes or high-pressure cleaners should be specified.
Cleaning time and cleaning cycles; Whereas for CIP and WIP systems the sequence of the individual cleaning sequences and their duration is established in the cleaning program, specifying times for the manual cleaning presents a certain problem, because the duration of the cleaning step is normally dependant on the visible result "clean" and not fixed to a specific time. Times specified for manual cleaning can even cause problems during the validation, as in practice, it is not always guaranteed that the times will be adhered to - due either to a lack of compliance with the given instructions or due to certain prerequisites not being in place (e.g. no clock in the wash room).
Rinsing and secondary treatment: More precise instructions regarding secondary treatment and drying of the equipment are given here. Whereas for CIP and WIP systems, very detailed instructions are possible (temperature and volume of the rinsing solution, number and duration of the rinsing sequences, air supply quantity and temperature during drying, duration of drying sequence), the instructions for manual cleaning are limited to a description of the working steps and aid used.
Assembly and disassembly: all assembly and disassembly procedures directly related to cleaning must be described in the cleaning SOP. This applies both to equipment parts and to parts of the CIP or WIP system.
Documentation
It should be specified where, how and by whom the cleaning will be documented, e.g.:
- entering details in the log book
- completing and affixing a cleaning label onto the equipment
- documentation in the batch record (previous and/or subsequent product) (see chapter 11.D.1 Cleaning procedure for rooms and chapter 11.H.2 Labelling in the manufacturing process)
Checks
The cleaning should be checked by a second person upon completion and the checks should be documented in a suitable manner. The checks can be made immediately after completing the cleaning and/or prior to re-use of the equipment.
8.B.3 Validating manual and automated cleaning
procedures
In contrast to automated cleaning, during which all the process parameters are integrated into the cleaning program in a defined and reproducible manner, manual cleaning uses a person - and therefore a factor which is very difficult to validate - as its variable.
How can these various starting situations be taken into account during the cleaning validation?
Standardisation and reproducibility of the process parameters
With regard to the standardisation and the reproducibility of the procedure - both important aspects of the validation - automated cleaning has a distinct advantage over manual cleaning. Process parameters such as sequence time, temperature, mechanical working time and cleansing agent dosage are defined in the program sequence and are therefore reproducible when the facility is operating without faults. In addition, the continuity of the cleaning procedure is also guaranteed. During manual cleaning, compliance with the above mentioned process parameters is, however, dependent on the reliability of the staff. Besides this the cleaning may be interrupted for various reasons, which may also affect the cleaning results. To increase the compliance of the staff, an awareness of cleaning validation requirements will need to be created in the form of training sessions. As cleaning is often seen as a simple or even a burdensome activity within the daily production routine, considerable training expenditure will be required. The fact that manual cleaning is often carried out by leasing companies, who frequently change their personnel, must also be critically examined. Regular training sessions and checks are indispensable in this case in order to ensure a professional and reliable implementation of the cleaning procedure with unwavering quality.
Compliance with process parameters
How can compliance with process parameters during validation of both automated and manual cleaning be ensured?
The use of the correct cleansing agent in the correct concentration can be guaranteed during automated cleaning procedures by a permanent feed and suitable control and monitoring of the dosage. With manual cleaning, misidentifications and deviations are more likely. These can be combated through suitable training, professional labelling and the existence of suitable dosage appliances.
The mechanics of cleaning can be well reproduced in automated processes. With manual cleaning, the devices to be used can be specified, but when implementing the cleaning, individual differences are unavoidable. Nevertheless, it should not be forgotten that manual cleaning is always linked to direct checks, meaning that a positive cleaning result then offsets the individual differences in the mechanical effect.
The temperature of the cleaning solution is easily reproducible for both processes, provided that hot and cold water are provided in the piping network at specified temperatures. If special temperatures which can only be produced by blending are required, temperature displays will be necessary.
Compliance with specified times is a weakness of manual cleaning, as - similarly to the mechanics - it is subject to individual deviations. It is also true, however, that direct checks on the success of the cleaning can offset this shortcoming. With automated cleaning, sequence times are fixed program parameters and can therefore be easily reproduced.
Volumes behave in a similar manner to time specifications. Whereas with automated cleaning the volumes are defined via flow rate and time, for example, and can therefore be reproduced, the specification of the quantity of cleaning solution and rinsing water for manual cleaning can often only be limited.
Also with regard to the sequence of the cleaning, i.e. the sequence of the individual working steps amd the continuity of the entire process, automated cleaning must be assessed in more favourable terms than manual processes, with regard to validation.
In view of these considerations, it is not surprising that the PIC/S guideline PIC/S PI 006 in section 7.5 Personnel has provided the following request: "It is difficult to validate a manual, i.e. an inherently variable/cleaning procedure.Therefore, operators carrying out manual cleaning procedures should be supervised at regular intervals."
Training and qualification
Whilst comprehensive and regular training is an indispensable prerequisite for a successful validation of manual cleaning procedures, the qualification of the cleaning systems and programs is an obligatory prerequisite for the validation of automated cleaning procedures (see chapter 4 Facilities and Equipment and chapter 6 Qualification).
The existence and correct installation of all components of the CIP or WIP system must be checked as early as the installation qualification (IQ) stage.
As part of the operational qualification (OQ) procedure, the actual functionality of the cleaning programs must be checked and guaranteed. It is not the success of the cleaning which is in the focus here, but the flawless sequence of the cleaning procedure in accordance with the specified cleaning program. The operational qualification of the cleaning program can therefore be carried out without a product.
Important test items for the operational qualification are, for example:
- functionality of the dosage pumps for the cleansing agent
- compliance with time specifications
- compliance with temperature specifications
- correct control and functioning of the spray nozzles
- constant pressure conditions in the piping system
Careful and comprehensive qualification therefore helps to prevent unwanted breakdowns and discrepancies during the cleaning validation.
|
Summary When optimising a cleaning procedure, the chemistry, temperature, mechanics and time process parameters must be harmonised in such a way that, in addition to the effectiveness of the process under worst case conditions, the cost effectiveness and validatability are also ensured. When compiling a cleaning SOP, a uniform standard should be laid down for all production equipment. This standard must contain a precise description of the equipment, an identification of the critical areas, a detailed description of the implementation of the cleaning and details on the documentation and checks of the cleaning. When validating manual cleaning procedures, regular and comprehensive training in addition to reinforced monitoring of staff are necessary prerequisites for the success of the validation. When validating automated processes, a careful and extensive inspection of the cleaning systems and programs as part of the installation and operational qualification is a necessary prerequisites for the success of the validation. |

