1.A Quality management in the pharmaceutical environment
Here you will find answers to the following questions:
|
1.A.1 Quality assurance in the GMP regulations
In addition to the requirements for proof and reproducibility of the product quality during manufacture and quality control, companies are obligated to implement a quality assurance system. The task of this system is to ensure that the drug products have the quality required for the intended use.
The requirements stipulated in the EU GMP Guideline are considered as the basic specifications which define precise expectations of the overall system and further specific proof requirements for the individual elements of the quality assurance system.
Chapter 1 of the EU GMP Guideline requires the holder of a manufacturing authorisation to manufacture drug products in such a way that their suitability for the intended use is guaranteed, they correspond to the requirements specified in the marketing authorisation and the patients are not put at any risk due to inadequate safety, quality or efficacy. The Guideline also lists the key points listed below, which must also be covered by the system.
Chapter 1 Quality assurance system
See also chapter C EU GMP Guide, chapter C.4 Part I Basic Requirements for Medicinal Products.
"The system of Quality Assurance appropriate for the manufacture of medicinal products should ensure that:
A. medicinal products are designed and developed in a way that takes account of the requirements of Good Manufacturing Practice and Good Laboratory Practice,
B. production and control operations are clearly specified and Good Manufacturing Practice adopted,
C. managerial responsibilities are clearly specified,
D. arrangements are made for the manufacture, supply and use of the correctstarting and packaging materials,
E. all necessary controls on intermediate products, and any other in-process controls and validations are carried out,
F. the finished product is correctly processed and checked, according to the defined procedures,
G. medicinal products are not sold or supplied before a Qualified Person has certified that each production batch has been produced and controlled in accordance with the requirements of the Marketing Authorisation and any other regulations relevant to the production, control and release of medicinal products,
H. satisfactory arrangements exist to ensure, as far as possible, that the medicinal products are stored, distributed and subsequently handled so that quality is maintained throughout their shelf life,
I. there is a procedure for Self-Inspection and/or quality audit which regularly appraises the effectiveness and applicability of the Quality Assurance system."
By definition, then, the primary task of the quality assurance system is to guarantee the quality of the manufactured and distributed products. But what does quality mean, from a pharmaceutical perspective (see figure 1.A-1)?
Quality |
|---|
Quality is the nature of a drug product, which is determined by the identity, content, purity, and other chemical, physical and biological properties or by the manufacturing procedure. |
This provides the link between the manufacture or manufacturing procedure and the quality assurance system and thus the product quality. The manufacturing procedure together with the quality control primarily determines the quality of the pharmaceutical products.
The quality assurance system establishes the conditions for this. The description of the quality assurance system in the EU GMP Guideline also stresses that the manufacture and quality control, and thus the EU GMP regulations, represent a significant part of the system. However, quality not only means the quality of the products, but also the quality of the necessary peripheral activities and accompanying operations. Here too, the EU GMP Guideline points out and prescribes that additional factors beyond the scope of this guideline, must also be implemented. Only then is a comprehensive quality assurance system introduced in the pharmaceutical company.
This means that there are several ways in which a pharmaceutical QM system can be set up. It can be based on the content of the processes to be described
- in accordance with the GMP rules and regulations (see chapter 1 of the EU GMP Guideline, Setting up a QM system in accordance with GMP), or
- in accordance with the quality management structure in line with DIN ISO, or
- using a combination of the two requirements
As there is no obligation to use one or other procedure in the pharmaceutical area, any combination of both variants is possible.
1.A.2 From quality assurance to quality management
Beyond the EU GMP Guideline, all regulations to be complied with stipulate that a company must set up and maintain a quality assurance system.
In modern times, however, the term should be extended to quality management system. This extension is required due to the active involvement of the management level. It is advisable to rename the term, as the quality assurance system alone cannot exist without active support from the management level. In almost all cases, the implementation of the system requirements requires money, time, staff and ultimately resources, and it can therefore only be supported by the company management (as management level). In principle, however, the step from a quality assurance system to a management system is not a recent invention. Rather, the involvement and responsibility of the management board was already provided for in the original definition in the EU GMP Guideline (see figure 1.A-2).
Participation of company management |
|---|
|
Principles The holder of a Manufacturing Authorisation must manufacture medicinal products so as to ensure that they are fit for their intended use, comply with the requirements of the Marketing Authorisation and do not place patients at risk due to inadequate safety, quality or efficacy. The attainment of this quality objective is the responsibility of senior management and requires the participation and commitment by staff in many different departments and at all levels within the company, by the company's suppliers and by the distributors .... (Chapter 1 EU GMP Guideline) |
In this case, responsible means that the company management must actively ensure that the objectives of all regulatory requirements that apply for the respective company (EU GMP Guideline, etc.) are completely and effectively implemented, and that the products do not have any defects. In further discussion, however, the structure of this active participation by the management level becomes problematic. The rules and regulations do not provide assistance for specific cases.
In the broadest sense, a management system means the implementation of systems that include the wider areas and departments - in addition to those specified in the guideline - in the quality management system. These departments include, for example, the research and development, engineering or marketing and sales areas, which were not or were only inadequately involved in a quality assurance system as previously interpreted. The tasks of these departments repeatedly fall within the GMP-regulated area.
Furthermore, external companies that make any kind of contribution to the product quality must also be included in the system (e.g. contract manufacturers, suppliers and service providers, see chapter 1.C.10 Qualification of suppliers and service providers).
The GMP rules and regulations may give information on the content, but they hardly provide any assistance on the structure of a quality management system. The points and implementation requirements listed in this chapter should help give a uniform understanding of the content of the quality management system and at the same time illustrate possible structures. The responsibilities, particularly at management board level, are also listed.
In this context, reference is made to the application of the management standard EN ISO 9001/2004:2000. This describes a comprehensive system for a company which, independent of the product quality to be achieved, defines all processes that are meshed and represent a modern quality management system together with the basic requirements of the GMP Guideline.
A pharmaceutical quality assurance system that comprises the standard requirements of EN ISO 9001:2000 together with the specifications of the EU GMP Guideline and a high level of involvement of the management level can be described as a complete and effective quality management system.
1.A.3 Position of quality assurance in the company
Here you will find answers to the following questions:
|
In the meantime, a quality assurance unit (Quality Unit, Quality Assurance, QA) has long been established in the pharmaceutical industry. This has the task of maintaining the quality management system. It regularly reports to the management board or the board of directors and is responsible for ensuring that the system works properly. The establishment of this unit is compliant with the requirements of the FDA, which also expects the existence of a Quality Unit. There are various possible structures for the area or department. According to a generally accepted understanding, quality control (QC department) can be integrated in it, or there can be two separate operational departments (QA department and QC department).
The following elaborations always speak of the Quality Unit, when the quality assurance department or a person with an equivalent function is meant.
1.A.3.1 Quality Unit as a staff function
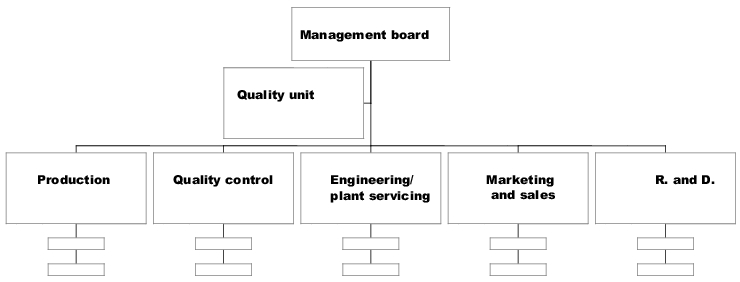
In order to meet the requirements in all areas and departments, and also to have the authority to issue directives in respect of the application and implementation of the quality management system, the Quality Unit as a staff function should report directly to the management board and should only be accountable to it.
|
This classification in the company organisation means the Quality Unit is efficient and capable of acting.
Figure 1.A-3 shows an example of a possible organisational structure and connection of the Quality Unit as a staff position. For simplicity's sake, only the departments that carry out GMP-regulated work are listed. The Quality Unit's responsibility in respect of the implementation and monitoring of the GMP requirements can also be extended to other areas or corporate divisions, such as registration or clinical research.
The organisation chart above shows the levels above and below the Quality Unit, and its reporting line. Together with the job description, it is possible to illustrate the responsibility and activities in relation to the authority to issue directives in other departments regarding quality management system requirements.
Organisational integration as a staff department should also show the responsibility and field of work of the Quality Unit for the other areas and departments, as the tasks and work of this department extend into nearly all areas and departments of the company. This can be illustrated in a responsibilities matrix, which shows the activities and responsibility of the Quality Unit in respect of GMP activities in relation to the areas and departments of the company.
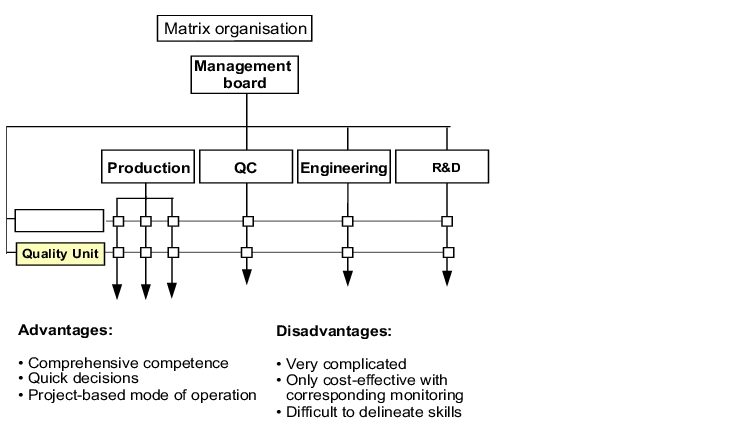
1.A.3.2 Quality Unit as a matrix function
Figure 1.A-4 shows the matrix function of the Quality Unit. This is an illustration of the elements of a quality management system, combined with the tasks of the Quality Unit in the individual areas. This illustration is a combination of the job description, the responsibility of the Quality Unit and the elements of the company-internal quality management system. In this illustration, the auditors can very quickly see the connection between all the above-mentioned points.
Department |
Manufacture |
Quality |
Research and |
Engineering |
Clinical |
|
|---|---|---|---|---|---|---|
Activity |
||||||
Documentation |
||||||
Risk management |
||||||
Qualification/validation |
||||||
Changes |
||||||
Deviations/OOS |
||||||
Complaints |
||||||
Corrective and Preventive Actions |
||||||
Qualification of suppliers/service providers |
||||||
Training |
||||||
Inspection |
||||||
Batch Record Review |
||||||
QM handbook/PIC |
||||||
R = Responsibility for implementation, I = Implementation of the activity |
||||||
This table should be drawn up in every company so that the questions raised during an audit or discussion can be answered. In the table, the individual activities should be marked with R = Responsibility or I = Implementation in the Quality Unit itself.
A different illustration of the matrix function is shown in figure 1.A-5, which is designed more in the form of an organisation chart. In this, the respective activities of the quality unit in the individual departments or areas can be illustrated by selecting the corresponding interfaces. The disadvantage of this illustration is that an exact specification of the work of the designated areas or departments is not possible.
|
In larger companies, there are now further substructures which exercise the activities of the Quality Unit in individual departments and areas. These are the so-called departments of a Quality Unit area. This extended structure is always expedient if the Quality Unit cannot carry out the full scope of the work required, whether due to capacity shortfalls or a lack of knowledge of the individual processes. The employees of the Quality Unit on-site are involved in the daily processes and activities, know the employees and their problems, and are able to intervene effectively immediately. In this structure, it is important that the reporting line, and also the disciplinary subordination, is still direct to the Quality Unit area. This rules out a direct influence by the on-site area manager, which under certain circumstances might not correspond to the objectives of quality assurance.
1.A.4 Responsibility of the Quality Unit
It is advisable to position the system responsibilities for all elements of the quality management system in this Quality Unit. System responsibility means that the employees in the Quality Unit control and guide the implementations of all tasks and processes from the quality management system and complete the processing of the necessary documents and thus also complete the operation. In this context, it is important to note that the Quality Unit employees only have a duty to provide assistance in the individual task areas, but do not have to actually carry out the activities themselves. Technically skilled processing should still be carried out by the respective specialist departments.
Ultimately, however, it is the legally responsible person or the Qualified Person who remains responsible for conformity in terms of the compliance of the individual operations with the regulatory requirements and in terms of specifications-compliant processing together with pertinent documentation. At most, the Quality Unit can make an active contribution to the achievement of these objectives, and provide support.
Here, the distinction between the function and responsibility of the Quality Unit is different than expected in America. Consider, for example, the responsibility for guaranteeing the validity of the manufacturing processes. In Germany and Europe, the head of production is responsible for ensuring that all necessary validations are carried out (see chapter 2 Personnel). The FDA, on the other hand, expects the Quality Unit to sign the validation report and release the validation.
Another example is that the Quality Unit in Europe is not responsible for releasing the starting materials/raw materials or the final products. It can provide assistance in releasing the validation documentation, but ultimately it is the head of production or the head of quality control who is legally responsible for correctness and for implementation of validation. There are other examples in which the FDA attributes responsibility to the Quality Unit, where the same responsibility is assigned to the legally responsible person or the Qualified Person in Europe. In these cases, quality assurance can carry out advance visual inspection and assessment, but in the end, the above-mentioned persons are responsible.
The key personnel listed in the EU GMP Guideline are not recognised by the FDA. In its understanding, the Quality Unit is responsible for all these functions.
In order to comply with both system requirements, both persons should sign the respective document, i.e. the head of the Quality Unit and the legally responsible person. Depending on the requirements, reference is made to the signature by the Quality Unit (USA), on the one hand, and to the signature by the legally responsible person or Qualified Person (Germany/Europe), on the other hand.
Ultimately, the head of the Quality Unit can be the Qualified Person or a top management employee (equivalent to the head of the Quality Unit), provided all functions are neatly separated from each other and stipulated in job descriptions. If you consider all the functions and requirements of the respective persons, it becomes apparent that there is a very high level of conformity between the activities. It is therefore not necessary to introduce new structures or responsibilities.
The Quality Unit only has system responsibility. That is, it must implement corresponding systems, establish written instructions, monitor compliance with the requirements and, in many cases, monitor or approve sub-steps.
In general, the processes in the Quality Unit for the quality management system can be described in the recurrent sequence specified here:
- Evaluation of missing systems/processes
- Formulation of solutions for the system
- Agreement of the solutions with the legally responsible person/management board
- Implementation of the solutions in the departments
- Coordination of the processes and responsibilities between the individual departments
- Monitoring of compliance
- Feedback to the management board
1.A.5 Tasks of a Quality Unit
The elements of a quality management system are not only limited to the individual chapters of the EU GMP Guideline. In the meantime, a new and modern understanding of the system has been established in pharmaceutical companies and the monitoring authorities. The primary task of the quality management system is to guarantee the quality of the products in manufacture and quality control, but also to take precautions in advance and initiate corresponding measures so that problems do not occur or cannot occur again.
Examples are the qualification of equipment and devices, as well as the introduction of a preventative system for maintenance and servicing of equipment and devices. These measures prevent deficiencies in the equipment and devices from being observed only after the manufacturing steps have been carried out, and thus calling the quality of the products into question only after the event. Rather, the regular and preventative maintenance and servicing of the equipment and devices ensures that, with all probability, they will not cause any problems during use in daily work. In addition to the many other operations and subsystems of the quality management system, this is just one example of the implementation of necessary preventive actions, in combination with monitoring of their compliance and implementation.
As the requirements of a quality management system have become more extensive in recent years, the individual departments and areas are no longer able to carry out all the tasks and measures. These tasks include monitoring measures which are better performed by an outsider.
In addition, there are tasks in the quality management system, which are to be completed comprehensively across all departments or areas, such as audits, training or documentation (see figure 1.A-6).
Quality assurance tasks |
|---|
|
For this reason, it is advisable to commission a department, which is completely independent of all areas, with the establishment and monitoring of the individual tasks. In the pharmaceutical company, the tasks and responsibilities must be clearly separated from each other, especially for persons with a legal or regulatory responsibility. The delineations are to be established in job descriptions or job specifications and signed by the management board and job holder as a sign of mutual agreement. In this connection, it is important that the responsibilities defined by the legislator are not mixed or passed on. An example of the job description for the head of the Quality Unit is given in figure 1.A-7.
Name of the job holder: |
Dept des.: xxx |
||
Manager function: |
Managing director/Board of directors Mr/Ms ... |
||
Number of subordinate employees: ... |
|||
The job holder shall ensure that 1. all relevant GMP principles for the defined tasks and obligations are taken into account and implemented to the necessary extent at company .... 2. the processes, systems and responsibilities of the quality management system are established and codified in a quality assurance manual. 3. the self-inspections in all functional units of the quality management system and the follow-up of the resulting agreed actions are carried out and the respective status determined, assessed and communicated in an appropriate manner. 4. the employees of company ... have the respective required level of training as regards GMP. 5. inspections by the authorities and supplier audits are professionally prepared and processed ("company escort"). 6. the documentation of the requirements documents is complete and current. 7. the management is informed of all quality-related demands. |
|||
The job requires the following employee qualification: |
|||
School education: |
A-level |
||
Vocational training: |
University degree as biologist, chemist, biochemist or pharmacist |
||
Professional duration: |
At least two years employment in the above-mentioned areas, preferably in quality assurance in the field of medicinal products or active pharmaceutical ingredients |
||
Specialist knowledge: |
Knowledge of the GMP rules and regulations, good spoken and written knowledge of English, management techniques, IT knowledge |
||
Combination of functions: |
A combination of functions with the head of quality control is possible with corresponding proof of suitability. |
||
Deputy regulation: |
The head of the Quality Unit is deputised by: |
||
Summary: The notion of quality assurance is firmly grounded in all pharmaceutical rules and regulations. In order to operate effective quality assurance, systems must be introduced to describe the basic procedures. Quality management only works with active support by the management board. The management must be assigned a responsible role in the establishment and maintenance of the quality management system. The Quality Unit can only be attributed a supporting role in the overall system. In this role, however, it has partial responsibilities which can be perceived in a staff function or in a matrix function. |